Take-aways:
- Genetic characteristics present at diagnosis in older patients with AML are the primary determinants of outcomes after transplantation.
- High-risk genetic features at diagnosis are associated with MRD positivity at first complete remission in these patients, but MRD positivity does not independently impact LFS.
- Attempts to eliminate persistent mutations by intensifying conditioning regimens may be “counterproductive” due to the toxicity risk.
Baseline genetic characteristics present at diagnosis are the primary determinants of allogeneic hematopoietic stem cell transplantation (HSCT) outcomes in older patients with acute myeloid leukemia (AML), according to a recent study. Moses Murdock, MD, of the Dana-Farber Cancer Institute, in Boston, Massachusetts, and colleagues, conducted the research and published their findings in Blood.
Older patients with AML tend to have an elevated risk of relapse and poor survival outcomes after allogeneic HSCT, which is often the only potential curative option for these patients. Multiple factors may contribute to the high relapse risk and poor survival outcomes after HSCT in these patients.
AML in patients aged 60 years or older is “enriched for high-risk cytogenetic abnormalities” and often “evolves from antecedent myelodysplastic syndromes (MDS),” Dr. Murdock and colleagues wrote. While myeloablative conditioning is typically used to lower the risk of relapse in younger patients with high-risk AML, older patients often undergo reduced-intensity conditioning to lower the risk of toxicity. However, older patients who receive reduced-intensity conditioning and have measurable residual disease (MRD) at their first complete remission (CR) have a higher risk of relapse.
This can complicate decisions about conditioning regimen strategies, but genetic analyses of diagnostic and CR samples from older patients with AML could identify prognostic features that may guide treatment decisions.
Study Design
Dr. Murdock and colleagues conducted a retrospective genomic analysis of diagnostic and CR specimens from a six-institution cohort of patients diagnosed with AML at age 60 years or older who underwent allogeneic HSCT during their first CR. The cohort did not include patients with acute promyelocytic leukemia or isolated extramedullary disease. The median patient age at diagnosis was 66 years (range, 60-76 years), and the median time from diagnosis to transplantation was 4.8 months (range, 1.9-21.0 months).
All 295 patients included had a banked bone marrow or peripheral blood sample collected before induction chemotherapy, and 65.1% had a sample collected in their first CR before transplantation.
The median time from CR sample collection to transplantation was 2.5 months (range, 0.1-14.0 months). Most patients who had a CR sample available received consolidation between their first CR and transplantation. The median time to relapse after transplantation was 4.8 months (range, 1.0-60.8 months). The median follow-up for survivors was 44 months (range, 6.7-155.3 months).
The diagnostic samples were analyzed for 113 genes “known to be recurrently mutated in AML or in germline syndromes predisposing to the development of myeloid malignancies,” Dr. Murdock and colleagues wrote. The remission samples were analyzed for 76 genes that are recurrently mutated in myeloid malignancies.
The study’s primary endpoint was leukemia-free survival (LFS), defined as the time from transplantation until death or AML relapse, whichever occurred first.
Study Results
Samples taken at diagnosis frequently included high-risk genetic characteristics. Somatic mutations indicating that AML evolved from antecedent MDS occurred in nearly half of patients (43.1%). Germline mutations and variants also occurred in diagnostic samples.
Certain genetic features at diagnosis significantly raised the risk of reduced LFS. Mutations in TP53 (hazard ratio [HR], 3.4; P<.001), JAK2 (HR, 2.7; P<.001), KRAS (HR, 2.0; P=.004), and FLT3 internal tandem duplications without a concomitant NPM1 mutation (HR, 2.2; P<.001) all significantly raised the risk of reduced LFS. However, DDX41 mutations were significantly associated with longer LFS (HR, 0.55; P=.036), as were mutations in NPM1 without concomitant FLT3 internal tandem duplications (HR, 0.56, P=.002).
The researchers used this data to generate a hierarchal model of molecular risk based on genetic characteristics at diagnosis. Unfavorable genetic characteristics were mutations in TP53 or JAK2 or FLT3 internal tandem duplications without a concomitant NPM1 mutation (n=63; three-year LFS, 6.7%). Favorable genetic characteristics were DDX41 or DNMT3A mutations or NPM1 mutations without a FLT3 internal tandem duplications, and no concomitant poor-risk mutations (n=95; three-year LFS, 62%). All other genetic characteristics were defined as intermediate genetic characteristics (n=137; three-year LFS, 45%; P<.0001, for the three-group comparison).
Most patients (79.7%) had at least one persistent mutation detected in diagnostic and remission samples, while 34.4% of patients had newly detectable post-treatment mutations that occurred in the remission sample but not the diagnostic sample. Nearly 40% of patients who had mutations in DNMT3A or TET2 at diagnosis had those mutations alone in their remission samples, while only 16.7% of patients who had an ASXL1 mutation had that mutation alone in their remission sample.
Patients with MRD positivity at their first CR had an increased risk of relapse and lower LFS compared with patients with DNMT3A or TET2 and MRD-negative
mutations. However, when the researchers used a multivariable model that accounted for patient risk factors at diagnosis, there was no independent impact of MRD positivity on LFS. This was “most likely because of its significant association with diagnostic genetic characteristics, including MDS-associated gene mutations, TP53 mutations, and high-risk karyotype,” Dr. Murdock and colleagues wrote.
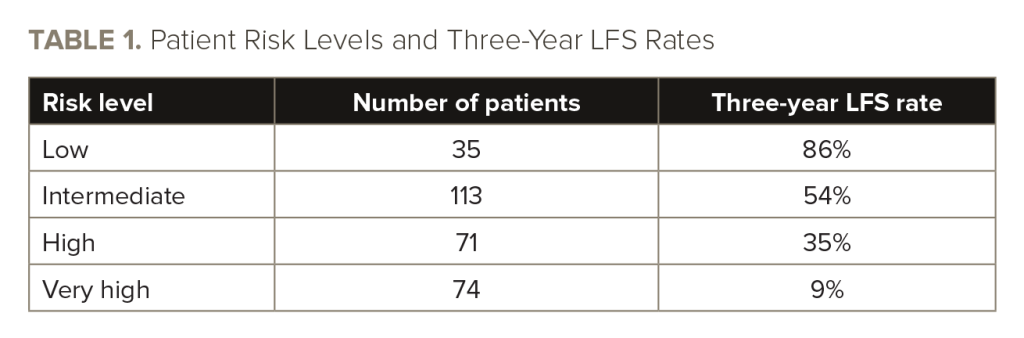
The researchers also designed a multivariable model to identify pretransplant factors that independently influence survival and relapse. The factors with a significant impact on outcomes were patient molecular risk, karyotype, HSCT comorbidity index score, complex remission with incomplete recovery, and clinically defined secondary AML. The researchers used the model to classify patients as low (n=35), intermediate (n=113), high (n=71), or very high (n=74) risk.
Low-risk patients had a significantly higher three-year LFS rate (86%) than intermediate-risk patients (54%; HR for death or relapse, 3.7; 95% CI, 1.8-11.3; P=.0004), high-risk patients (35%; HR, 5.6; 95% CI, 2.6-17.7; P<.0001), and very high-risk patients (9%; HR, 13.4; 95% CI, 6.3-42.2; P<.0001). See TABLE 1 for an overview of patient survival outcomes by risk level.
The three-year LFS rate was 42.1%, and the three-year overall survival rate was 46% for the entire cohort. The three-year cumulative relapse rate was 38%, and the three-year non-relapse mortality rate was 29%.
Limitations and Conclusions
The study’s limitations included its retrospective nature and the potential heterogeneity introduced by the timing of sample collection from patients.
“As such, use of this baseline model to guide clinical decision making for older patients with AML will require validation by subsequent prospective studies of cohorts with uniform prior treatment histories and sample collection time points,” the investigators wrote.
However, the findings can still inform clinicians about the relative risks and benefits of potential treatment strategies for this population of patients.
“Nevertheless, our results indicate that most of the post-transplant relapse risk in older patients with AML is encoded at the time of diagnosis and that the likelihood of molecular MRD positivity in these patients is primarily a reflection of baseline genetic risk,” Dr. Murdock and colleagues concluded. “Attempting to eradicate persistent AML mutations with dose intensification may be counterproductive in older patients, given their high risk of treatment-
related toxicity.”
Reference
Murdock HM, Kim HT, Denlinger N, et al. Impact of diagnostic genetics on remission MRD and transplantation outcomes in older patients with AML. Blood. 2022;139(24):3546-3557.






 © 2025 Mashup Media, LLC, a Formedics Property. All Rights Reserved.
© 2025 Mashup Media, LLC, a Formedics Property. All Rights Reserved.