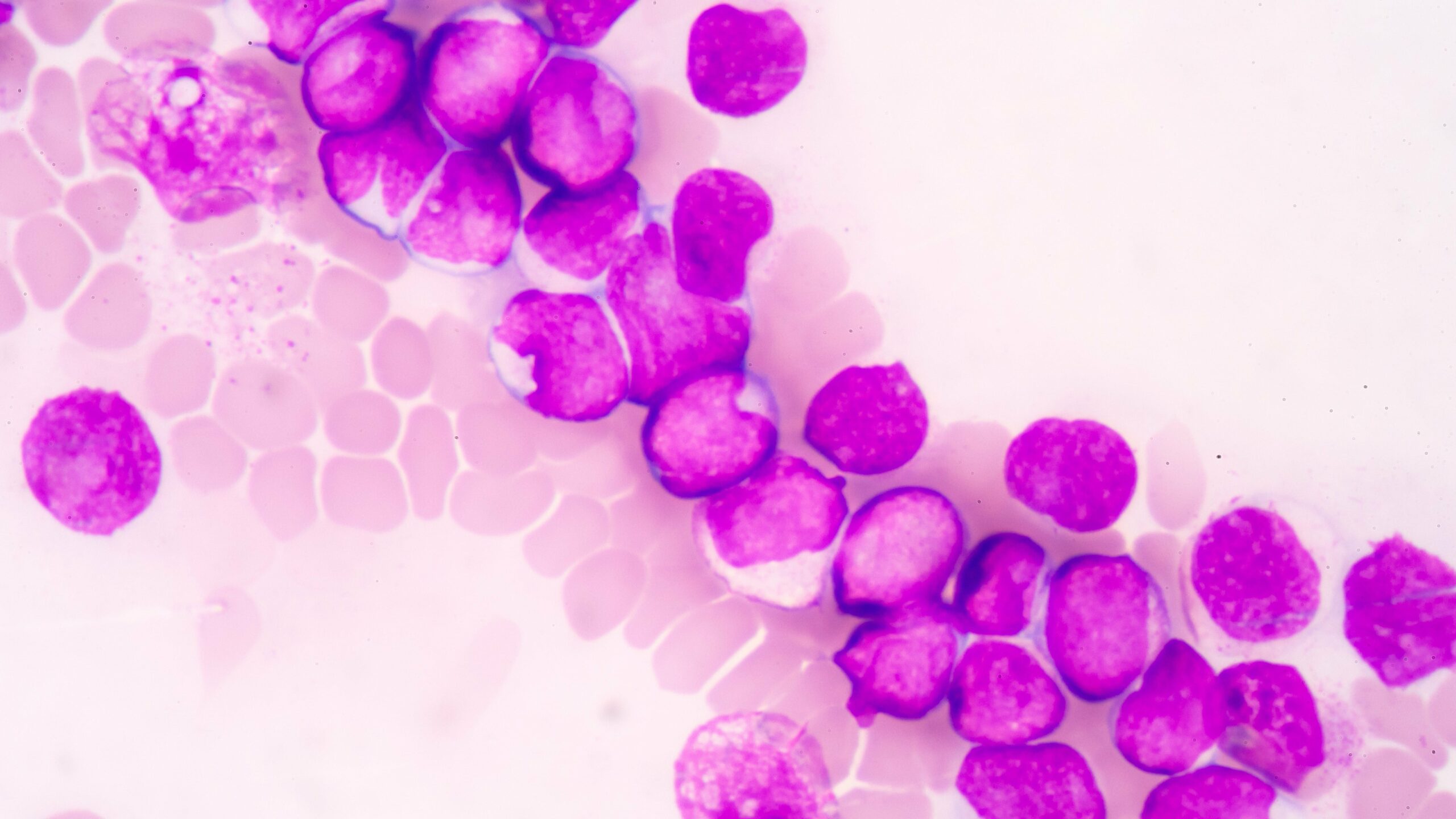
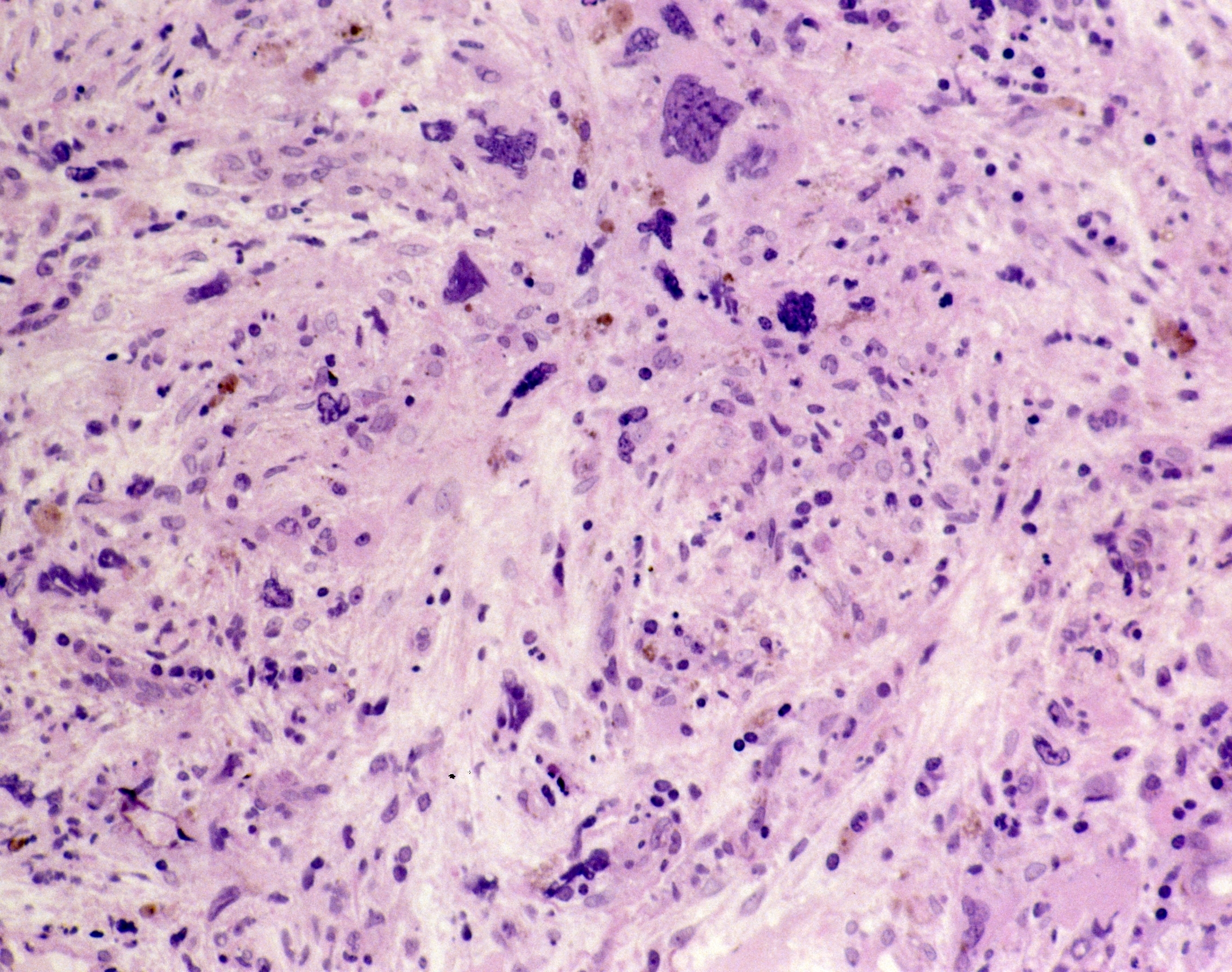
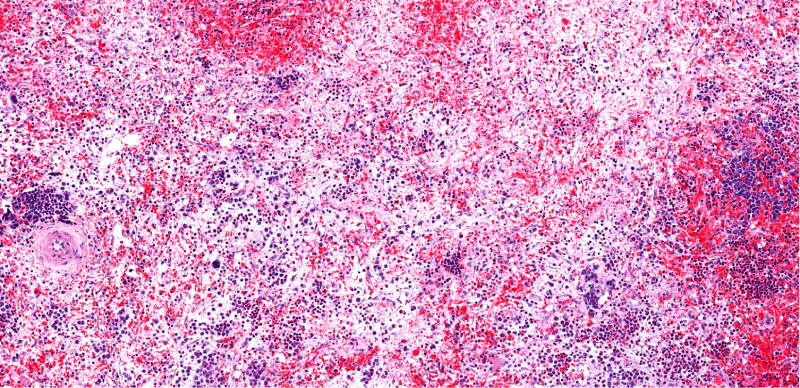
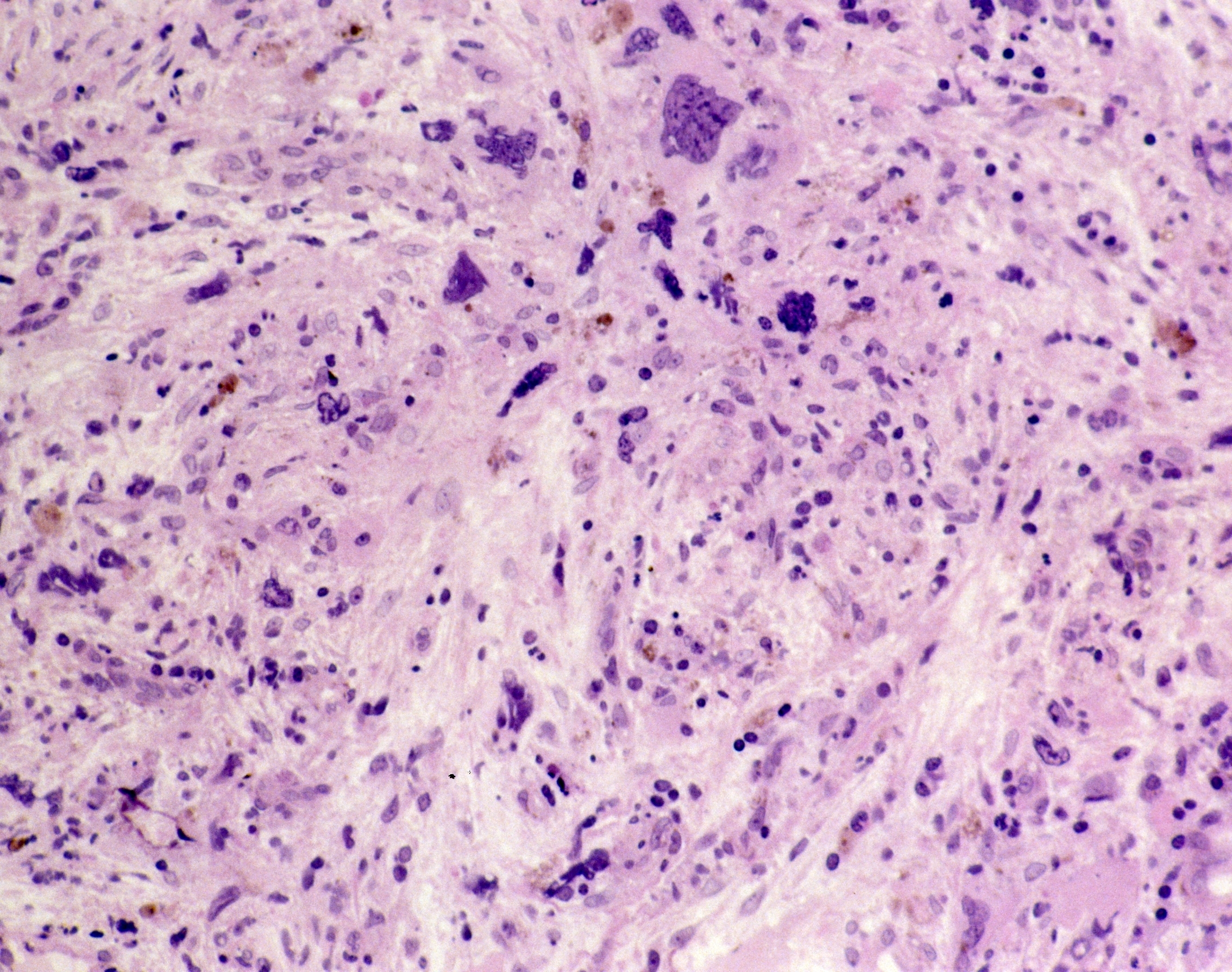
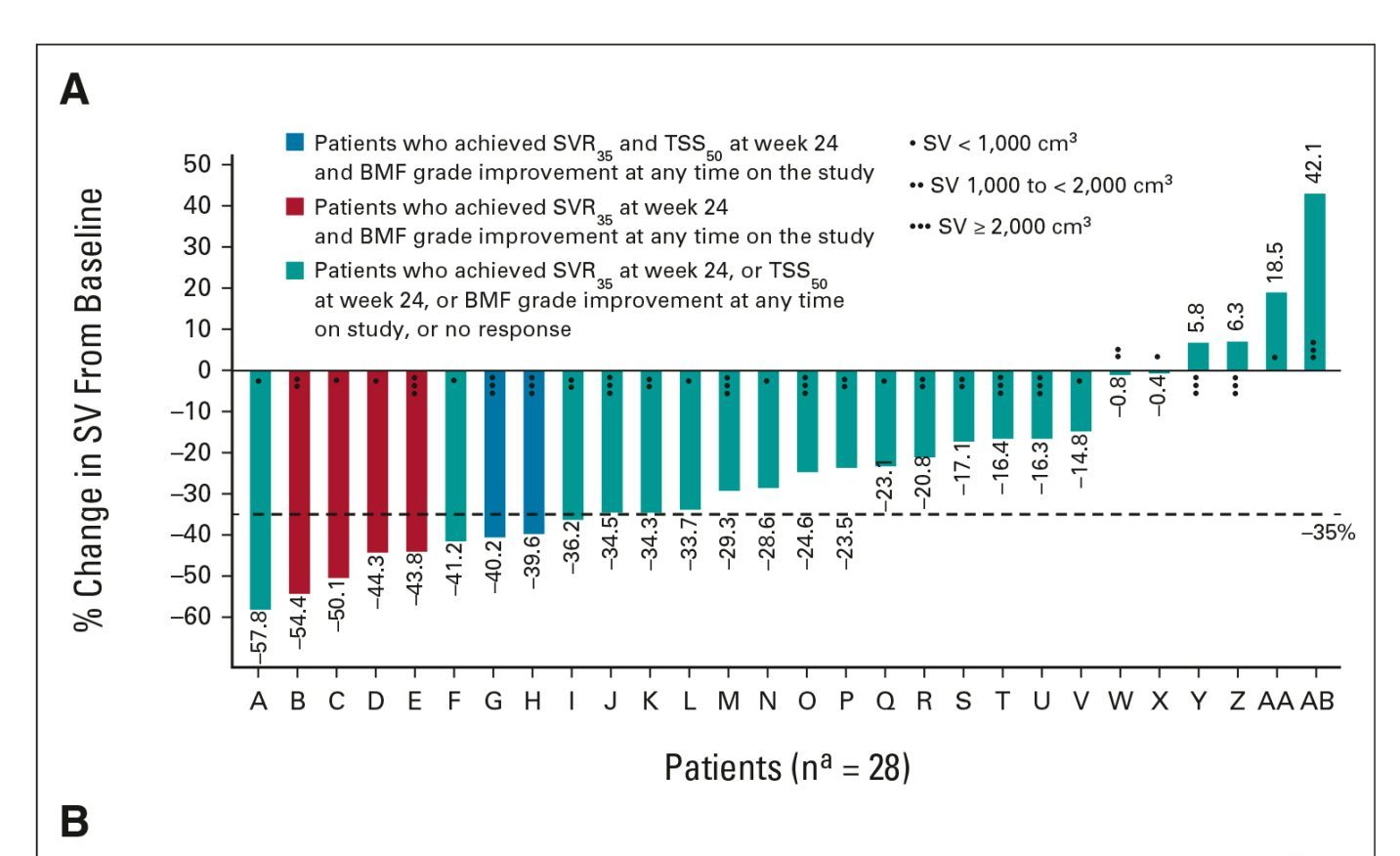
Myelofibrosis
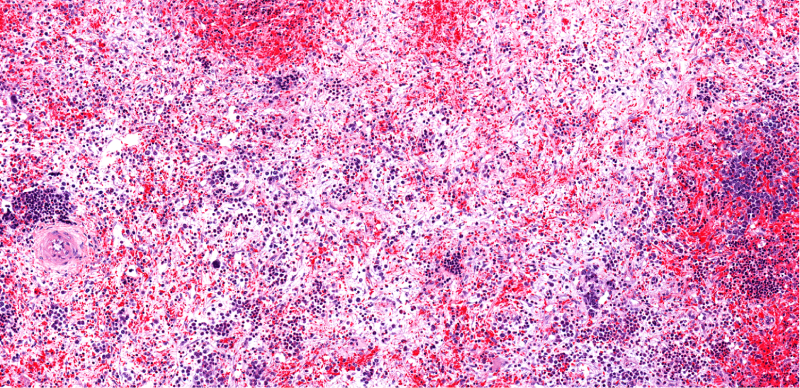
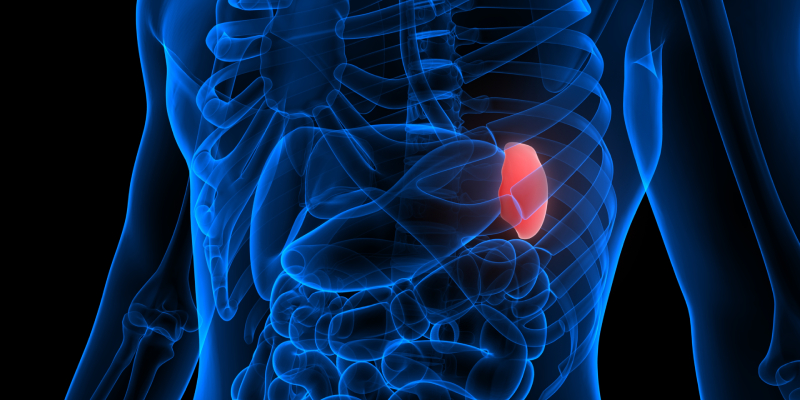
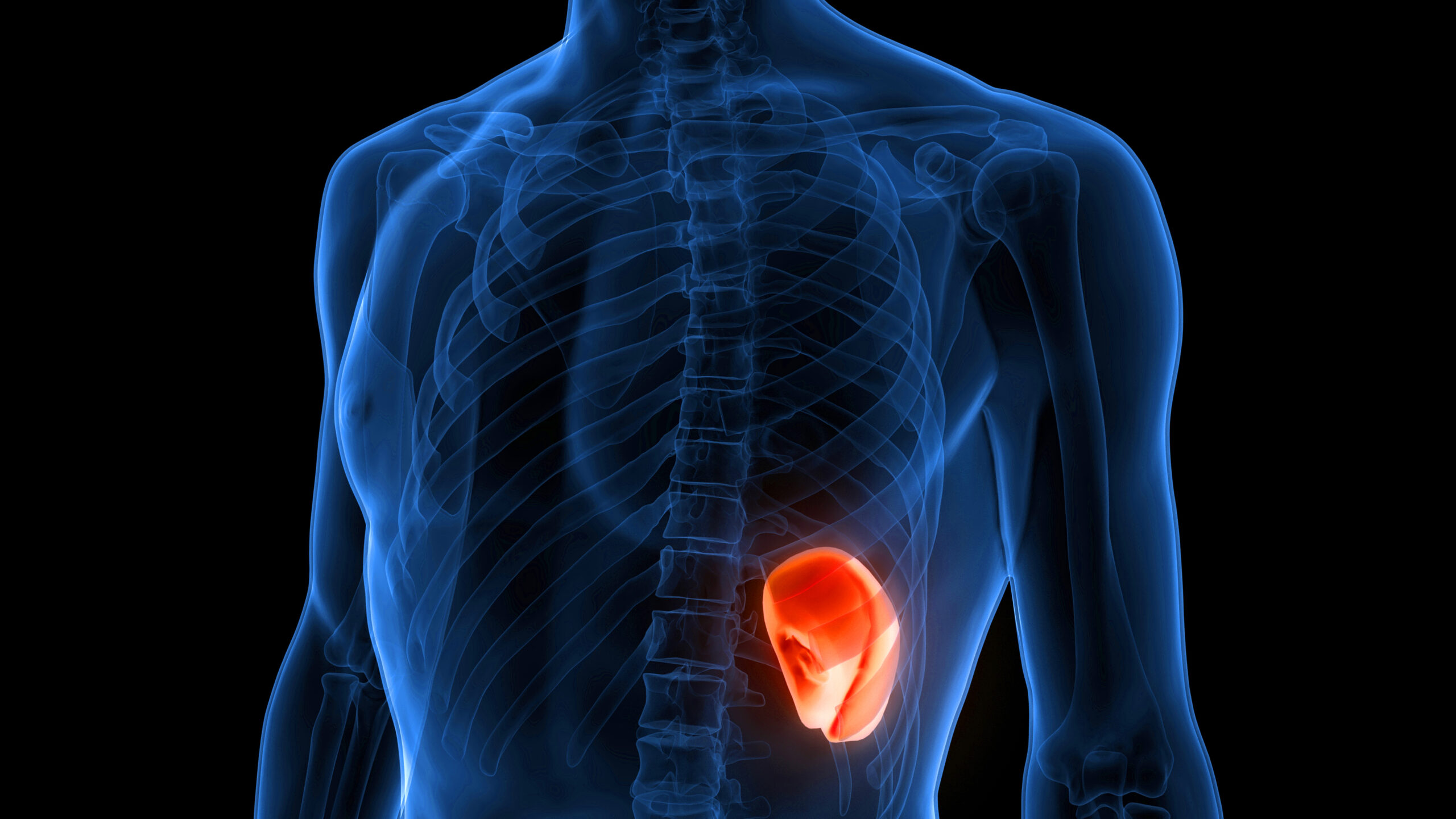
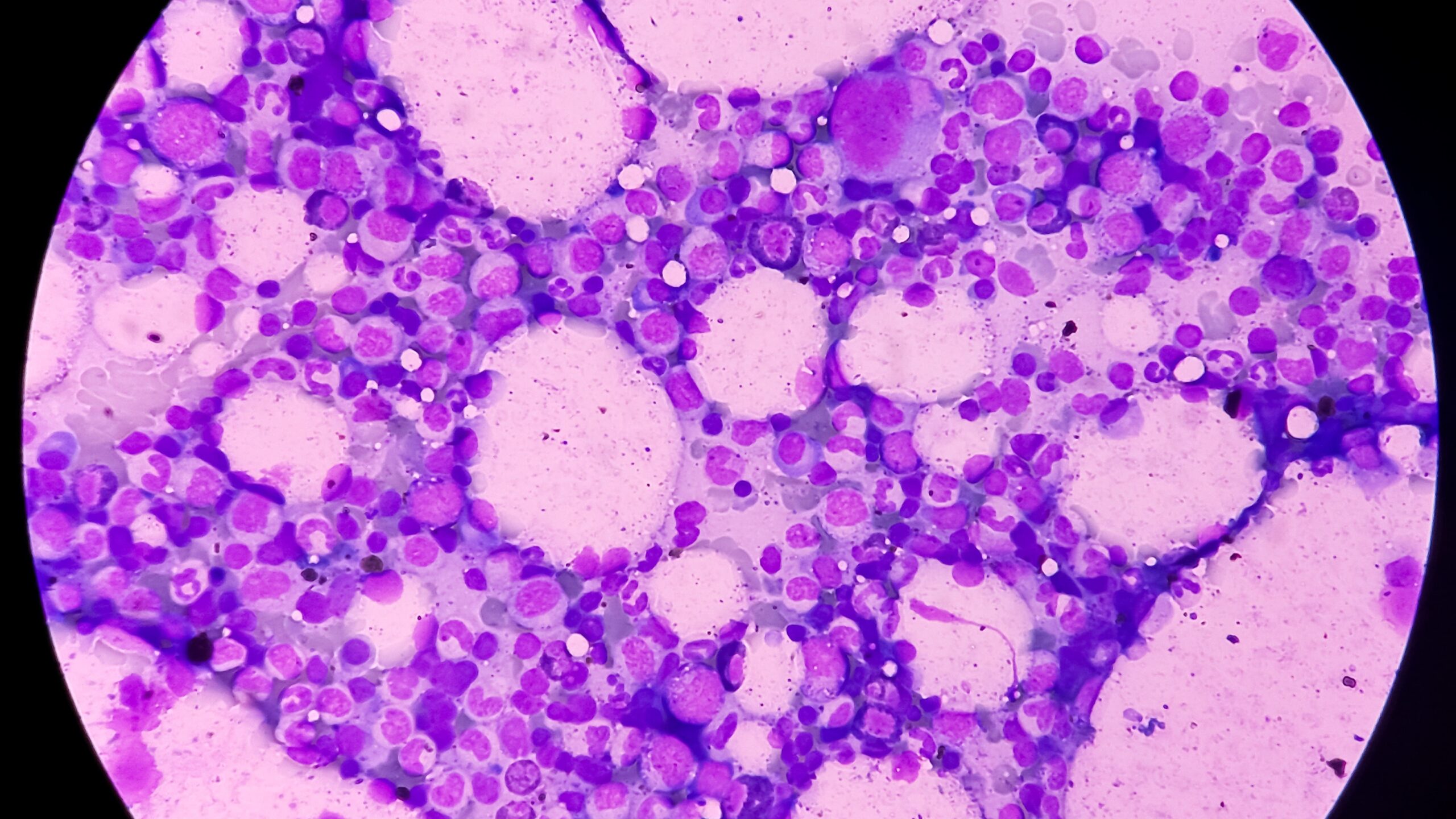
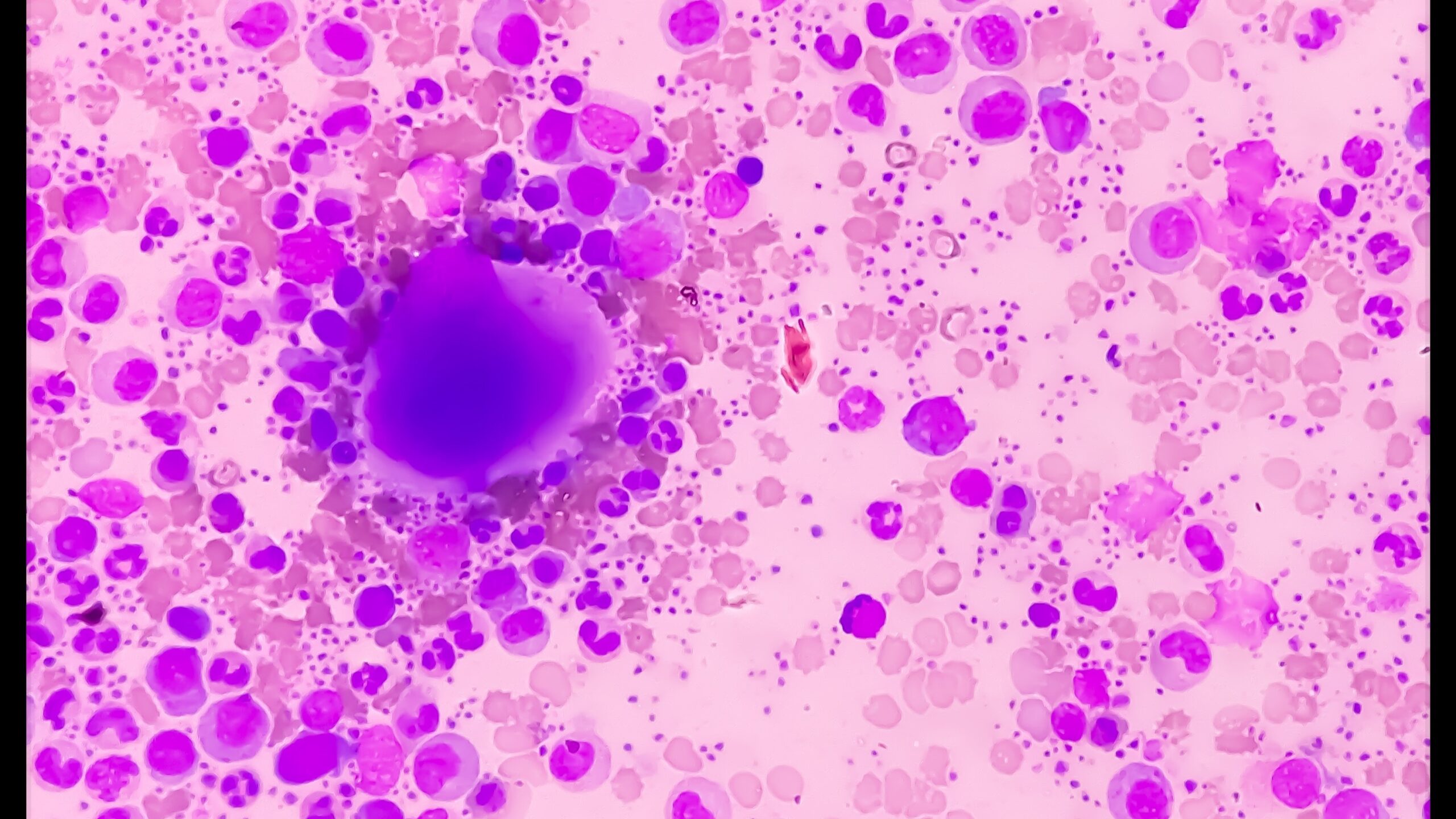
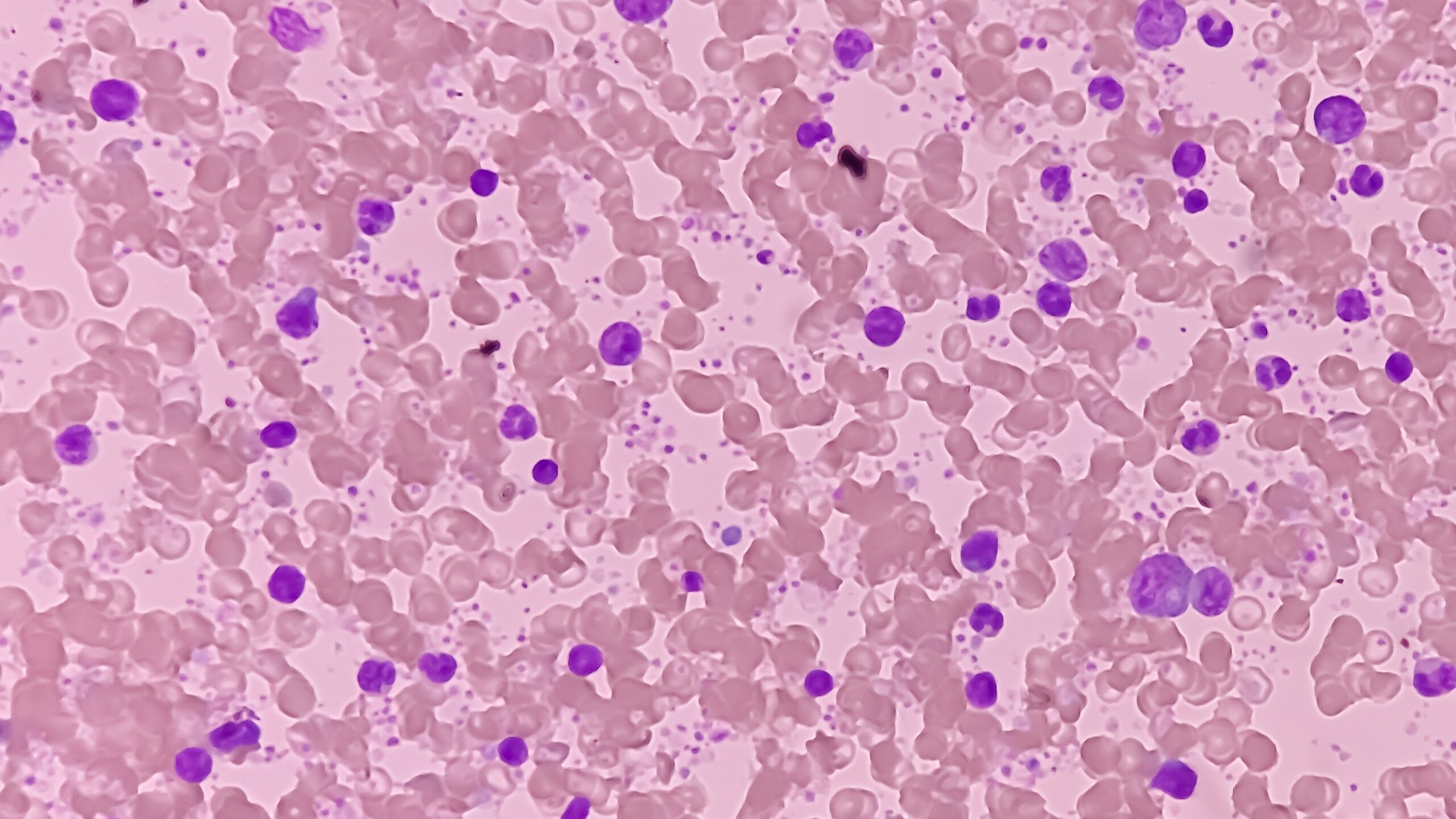
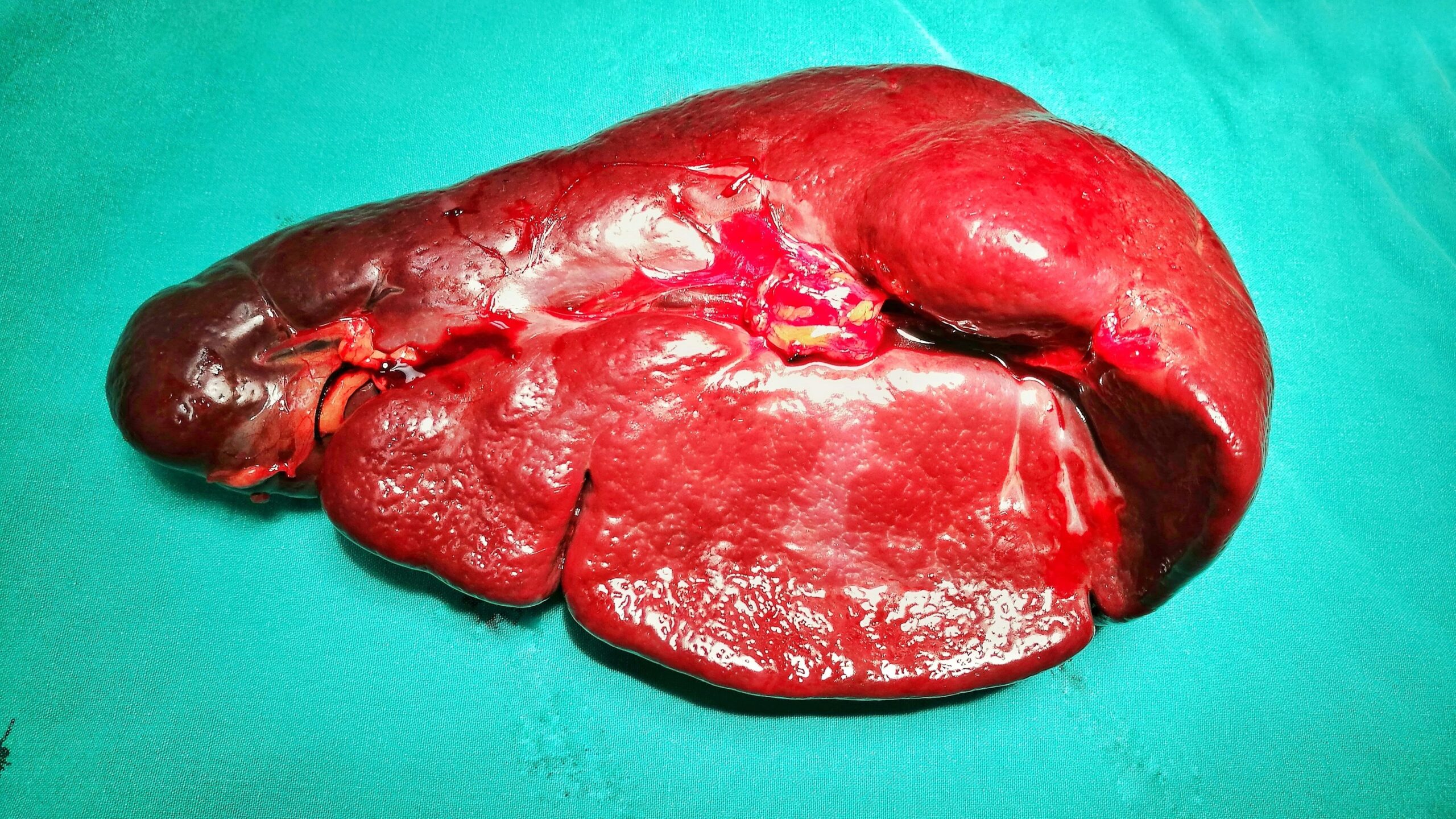
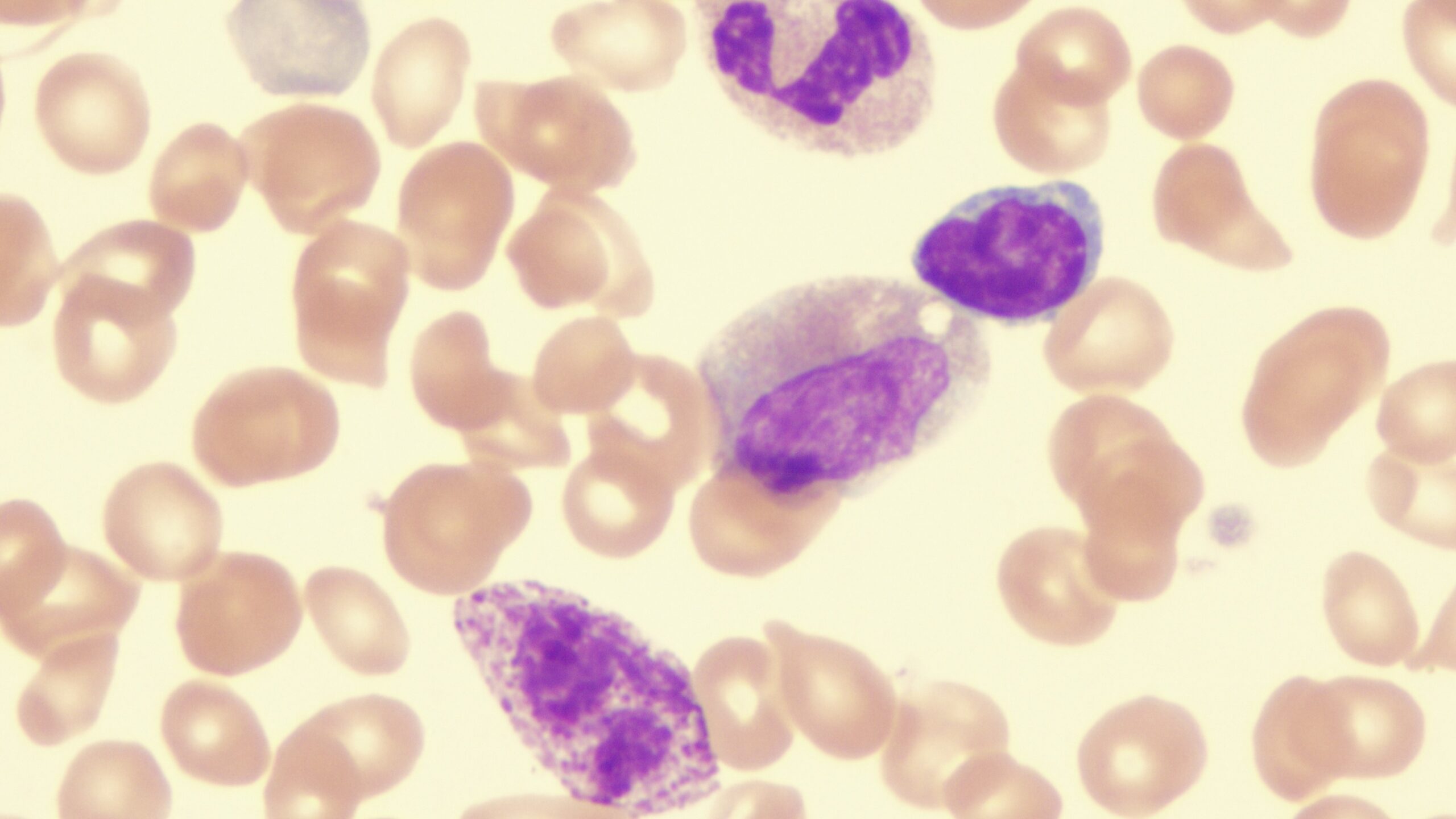
Myelofibrosis (MF) is a rare blood cancer that leads to scarring in the bone marrow, which prevents the normal production of blood cells. The condition is a primary subtype of myeloproliferative neoplasms (MPNs) and, in some cases, starts as another MPN. Risk factors for primary MF are unclear, although a history of polycythemia vera (PV) or essential thrombocythemia (ET) are risk factors for development of secondary MF. The disease is stratified as low, intermediate, or high risk using various International Prognostic Scoring System scales, and the prognosis depends on individual risk factors including age, comorbidities, and treatment response.
































































































 © 2025 Mashup Media, LLC, a Formedics Property. All Rights Reserved.
© 2025 Mashup Media, LLC, a Formedics Property. All Rights Reserved.