Transplantation & Cellular Therapy
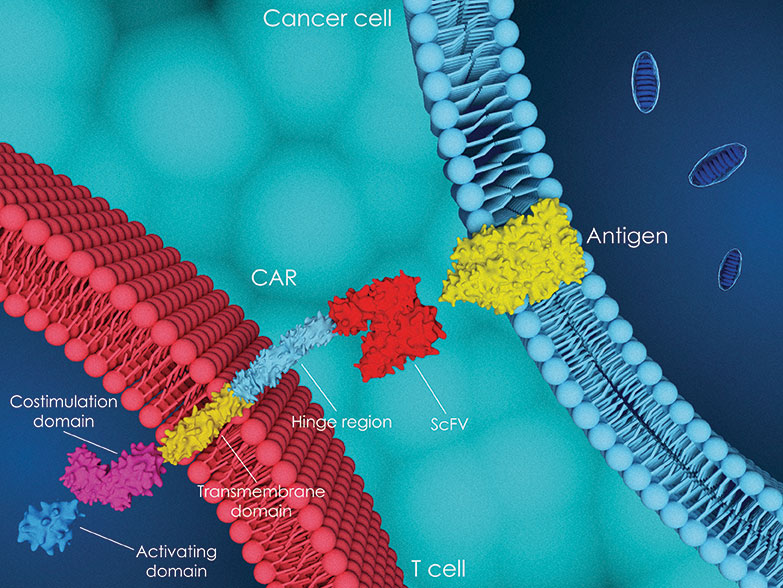
The latest news, research, and perspectives in transplantation and cellular therapy. Autologous and allogeneic hematopoietic stem cell transplantation represent potentially curative options for some patients, while the field of non-transplant cellular therapies, such as chimeric antigen receptor (CAR) T-cell therapies, CAR natural killer cell therapies, and genetically modified T-cell receptors, is also expanding to offer more patients curative options.







































































































 © 2025 Mashup Media, LLC, a Formedics Property. All Rights Reserved.
© 2025 Mashup Media, LLC, a Formedics Property. All Rights Reserved.