MPN
Myeloproliferative neoplasms (MPNs) are rare cancers and include diseases of the blood and bone marrow. MPNs involve dysregulation at the multipotent hematopoietic stem cell (CD34). MPNs occur when the bone marrow produces too many red blood cells, platelets, or certain white blood cells. The primary subtypes include myelofibrosis, polycythemia vera, and essential thrombocythemia.
Gaps in coordination and collaboration between primary care and oncology may impact long-term survival outcomes for patients.
In the SURPASS ET trial, ropeginterferon alfa-2b was compared to anagrelide in second-line management of ET.
Dose escalation study data presented at EHA2025 showed durable response in patients with disease resistant to prior therapy.
The agent, nuvisertib, previously received Orphan Drug Designation from the FDA for this indication in May 2022.
The phase 3 SURPASS-ET trial presented at ASCO evaluated ropeginterferon alfa-2b versus anagrelide for the treatment of ET.
Expert insights from a HemOnc Pulse Live! session offer a compelling look at where CML and MPN care is headed.
How have TKIs transformed CML survival and what’s next for treatment-free remission and deeper MPN responses?
100% MRD-negative CR in Ph+ ALL? Dr. Catherine Lai recaps standout abstracts from an ASCO rapid oral session.
Gecacitinib improved spleen volume, symptoms, and anemia in JAK inhibitor–naive MF, regardless of baseline anemia severity.
The MANIFEST-2 trial compares pelabresib plus ruxolitinib with placebo plus ruxolitinib in JAK-inhibitor-naive myelofibrosis.
Interferon improved myelofibrosis-free survival in adolescent and young adult patients with polycythemia vera.
Experts discuss early detection in myeloid malignancies, from current challenges to future innovations.
Studies have shown that oncologists are leaving the workforce at increasing rates in association with growing workloads.
This agent also has Orphan Drug designation from the EMA and a phase III study is currently enrolling patients.
Experts discuss patient-centered approaches and novel therapies reshaping MF treatment in a roundtable.
Experts explore anemia therapies, JAK inhibitors, and transplant innovations shaping the promising future of MF care.
Experts discuss molecular insights into MF, highlighting mutations, risk assessment, and evolving therapeutic approaches.
Experts discuss current treatments and ongoing research in myelofibrosis, highlighting new therapies and clinical trials.
Experts discuss CALR-targeting therapies, symptom assessment, and the future of myelofibrosis treatment and regulation.
LucyRx’s pharmacy network includes Northwestern Medicine Specialty Pharmacy, Dana Farber Cancer Institute, and more..
The ASCO Association Board Chair has written letters to the new heads of the CMS, FDA, and NIH.
The Community Oncology Alliance outlined solutions for Congress to address systemic challenges of the U.S. healthcare system.
More than 240 meetings were held with members of Congress as part of the ASCO 10th Annual Advocacy Summit.
Investigators observed that overall treatment has improved but hospitalizations and other events indicate unaddressed need.
Harboring high molecular risk mutations and a lower JAK2V617F VAF were independent adverse prognostic factors for survival.
The Myelofibrosis Symptom Assessment Form is the only instrument currently available for evaluating myelofibrosis symptoms.
According to John O. Mascarenhas, MD, the phase 3 MANIFEST study heralds a new era of combination therapy for myelofibrosis.
In a phase 2 trial the hepcidin mimetic increased hematocrit control while reducing phlebotomy and patient-reported symptoms.
Most patients with JAK2, CALR, and MPL mutations had mutation clearance 100 days after HSCT.
A study found limits to the prediction utility of thrombosis history in patients with PV and pulmonary hypertension risk.
Melody Smith, MD, MS, shares data on burnout in hematologist oncologists, why it occurs, and how to prevent it.
Andrew Kuykendall, MD, presented on the status of myelofibrosis treatment in the era of 4 approved JAK inhibitors.
MRD testing before HSCT may offer insight into survival outcomes for patients with MDS/MPN.
Discussing the evolving role of selinexor in treatment of myeloproliferative neoplasms.
Watch as Viges and Dr. Mascarenhas explore strategies to enhance patient-centered care in MPN management.
Exploring the latest advancements in myelofibrosis research with Kapila Viges, MD and John Mascarenhas, MD.
Two experts discuss the role of surrogate end points in MPN clinical trials.
A new study has identified genetic factors that can affect the molecular pathways involved in treatment response.
A common treatment goal for polcythemia vera is to control HCT with therapy, phlebotomies, and cytoreductive therapies.
Issues addressed include mandatory versus optional biopsy, informed consent, and safety in both adult and pediatric patients.
VGT-1849A is aJAK2 inhibitor that is designed to have less off-target suppression of JAK1, JAK3, TYK2, and other kinases.
SURPASS-ET compared second-line treatment with ropeginterferon alfa-2b with anagrelide in patients with ET.
The panel moderated by Prithviraj Bose, MD, goes into detail on practical aspects of this agent's use in the clinic.
The expert panel moderated by Dr. Bose looks at noteworthy study work on use of this agent for anemia in myelofibrosis.
Empaneled experts describe their investigations of momelotinib in a continued discussion moderated by Prithviraj Bose, MD.
The expert panel moderated by Prithviraj Bose, MD, tells which research presented at the Meeting they found most exciting.
Esteemed experts provide valuable insights into the evolving myelofibrosis landscape and its implications for patient care.
Key takeaways from the top ASH abstracts in the myelofbrosis space.
A roundtable discussion starts with a conversation about the myelofibrosis treatment landscape.
An expert's insights on the latest advancements in MF treatment presented at the ASH 2024 meeting.
There may be a link between obesity and how myeloproliferative neoplasms develop, research shows.
Outcomes for patients with AML and MDS who experience relapse after alloHSCT are poor.
Overall, fedratinib 400 mg daily was a safe MTD for post-HSCT maintenance therapy for participants with MPN.
Discussing current clinical trials iand unmet needs in myelofibrosis.
Interim results suggest acceptable safety and high efficacy of CPX-351 plus ivosidenib.
Findings suggest a potential metabolic benefit of pacritinib, warranting further investigation in myelofibrosis.
The PROMise trial aims to examine the safety and preliminary efficacy of OPN-2853 with ruxolitinib for MF treatment.
HMA plus venetoclax may achieve higher response rates and improve event-free survival in high-risk MDS.
In a murine mitochondrial succinate dehydrogenase knockdown model of low-risk MDS luspatercept alleviated anemia.
Odyssey is an ongoing, open-label, phase 2 study that will assess the benefit of adding luspatercept to momelotinib.
At ASH 2024, updated results of the RESTORE trial were presented.
These real-world findings highlight momelotinib as an effective and practical treatment for managing MF in everyday practice
BOREAS is the first global phase 3 study to demonstrate clinical efficacy of a single-agent treatment for r/r myelofibrosis.
Hypomethylating agents, such as azacitidine, are the standard-of-care treatment for MDS.
John Masarenhas discussed the development of the human telomerase inhibitor imetelstat for the treatment of myelofibrosis.
Improvements in the frequency of transfusions and of anemia symptoms are interventional priorities for myelofibrosis.
A phase 2 trial showed selinexor was well tolerated, and further studies are investigating its efficacy in other MF settings.
Ruxolitinib is the standard of care for patients with MF; however, it does not address the significant anemia burden.
A retrospective, real-world analysis shows momelotinib reduces anemia in patients with myelofibrosis.
A phase 1/2a study is investigating the safety and efficacy of PXS-5505 in myelofibrosis.
What potential does ASTX727 have in MDS and AML treatment?
Ivosidenib is a promising oral therapy for patients with IDH1-mutated MDS.
HOPE-PMF will recruit 150 participants with prefibrotic PMF or PMF at low or intermediate-1 risk.
Study findings indicate that allo-HSCT may offer a potentially curative treatment for high-risk BP-MF patients.
The safety was improved and the symptom burden of MF was reduced and remarked that the high burden of inflammation.
Benefits were observed of flonoltinib maleate in myelofibrosis were observed regardless of prior JAK inhibitor exposure.
Harinder Gill, MBBS, MD et al deems bomedemstat/ruxolitinib safe and tolerable for second-line MDS treatment.
Patients in a phase 1 study saw remarkable symptom improvements with INCB057643 and tolerated treatment well.
Hispanic ethnicity and prior transplants mismatched unrelated donors were prognostic of worse outcomes in older MF patients.
A trial in progress, phase 3 IMpactMF trial, investigates the encouraging efficacy/safety of imetelstat in MF.
Pacritinib demonstrated superiority to best available therapy for spleen volume reduction, total symptom score, and more.
Patients with splenomegaly are more likely to be referred for HSCT.
Selinexor plus ruxolitinib was well tolerated, reduced symptom burden, and led to spleen volume reduction.
One year of pacritinib treatment stabilized or improved thrombocytopenia and anemia in patients with myelofibrosis.
The phase III PERSIST-2 study compared symptom results from pacritinib with those of best available therapy and ruxolitinib.
Patients' genomic profiles and baseline laboratory values have implications for their treatment outcomes with these agents.
The International Prognostic Scoring System, Dynamic International Prognostic Scoring System, and other models were applied.
A retrospective study found momelotinib plus pacritinib led to reduced need for red blood cell transfusion in patients.
A real-world retrospective study evaluated transfusion dependent or nondependent patients at ruxolitinib initiation.
Management of asymptomatic, clinically stable disease is not necessarily improved by implementing routine hemostasis tests.
Investigational agent DISC-0974 produced anemia response independent of patient transfusion dependency or JAK inhibitor use.
A study observed that addition of pelabresib to ruxolitinib led to bone marrow microenvironment improvement.
An expert on myelofibrosis comments on promising results from current trials and on the need for useful clinical biomarkers.
History of thrombotic events, hematocrit ≤0.45 L/L, and JAK2 p.V617F were identified as risk factors for progression.
LOXL2 upregulation is associated with key inflammatory signaling pathways in primary myelofibrosis.
Treatment with selinexor reduced hepcidin and pro-inflammatory cytokines in patients with myelofibrosis.
The rates of baseline comorbidities and constitutional symptoms were higher in patients with anemia versus those without.
New or worsening anemia following initiation of ruxolitinib does not appear to diminish clinical benefit.
Hemoglobin improvement at week 24 after transfusion is associated with improved HRQOL in myelofibrosis and anemia.
Prospective analysis suggest that 4.3% of patients with ET progressed to myelofibrosis over five years of follow up.
Patients with MF or ET with CALR or JAK2 mutations carried similar symptom burdens according to prospective data.
The phase III MANIFEST-2 trial compared pelabresib plus ruxolitinib with placebo plus ruxolitinib in JAKi-naive patients.
The findings were from a retrospective study of patient data from the US Flatiron Health electronic health record database.
A retrospective analysis covered two phase III trials of the JAK2 inhibitor in patients with and without monocytosis.
A study evaluated progression according to hemoglobin levels, platelet counts, constitutional symptoms, and other markers.
CTLA-4 blockade increased the cytotoxic T-cell–mediated immune response against myelofibrosis cells in human xenografts.
A Markov model has compared delayed with immediate performance of allogeneic HSCT following ruxolitinib failure in PMF.
Using mass spectrometry-based proteomics, chemokine platelet factor 4 (PF4) was identified as a driver of myelofibrosis.
New therapies, such as TP-3654, are currently being explored in combination studies.
Three of four patients with myelofibrosis had objective responses at one year, and no dose-limiting toxicities were observed.
No significant differences in HSCT were observed, suggesting haploidentical HSCT as an acceptable treatment approach.
AI-driven quantitative analysis was compared with manual evaluation of bone marrow trephine slides in a phase II trial.
Julie Braish, MBBCh, discusses her study on JAK2 allele burden in myelofibrosis presented at ASCO 2024.
Dr. Rampal, of Memorial Sloan Kettering Cancer Center, discusses his abstract at the EHA 2024 Congress in Madrid, Spain.
MPN depend on mutated JAK signaling, and targeting the mutations suppressed disease features and improved overall survival.
Pelabresib plus ruxolitinib improved spleen and symptom responses in JAKi-naïve patients with myelofibrosis.
Treatment with luspatercept improved transfusion burden and anemia across four cohorts of patients with myelofibrosis.
Prefibrotic PMF is an MPN with distinct characteristics comprising histopathological, clinical, and biological parameters.
Prefibrotic myelofibrosis is the focus of “SOHO State of the Art Updates and Next Questions.”
Momelotinib achieved higher rates of transfusion independence compared with ruxolitinib.
Splenic irradiation before HSCT is associated with reduced spleen size and lower incidence of relapse in patients with MF.
Sotatercept monotherapy, and combined with ruxolitinib, is a safe and effective treatment for patients with PMF and anemia.
Raajit Rampal, MD, PhD, and the "Blood Cancer Talks" hosts discuss the diagnosis and management of essential thrombocythemia.
The primary endpoint was overall best response after blast-reduction therapy.
Momelotinib and ruxolitinib improved bone marrow fibrosis, but changes were not associated with improved outcomes in MF.
Dr. Harrison discusses her current clinical research, building community with MPN Voice, and her journey into hematology.
A drug approval specific to CMML would catalyze a surge of interest in the hematology-oncology community.
Nicolaus Kröger, MD, discusses a study on GVHD and its impact on relapse in patients with myelofibrosis undergoing HSCT.
Myelofibrosis, a rare blood cancer, affects four to six per 100,000 individuals in the United States.
An international working group collaborating with the EBMT and ELN updated the 2015 guidelines for HSCT in myelofibrosis.
Ruxolitinib plus pelabresib was well tolerated and improved spleen and symptom burden in patients with myelofibrosis.
Parsaclisib plus ruxolitinib can improve symptoms and spleen volume in certain patients with myelofibrosis.
Sanjay Patel, MD, discusses a study on spatial mapping of human hematopoiesis using bone marrow tissue.
FREEDOM2 is a phase III study comparing fedratinib with best available therapy as a second-line treatment for myelofibrosis.
“Among the high-risk splicing mutations, SRSF2 has the worst prognostic role,” Dr. Braish and colleagues wrote.
The REVEAL study identified five risk factors, including leukocytosis, duration of time with PV, and more.
The treatment led to reduced hepcidin levels when used alone or in combination with ruxolitinib.
Venugopal highlights myelofibrosis research at ASH 2023.
Real-world databases lent insight into how often patients with PV experience thromboembolic events.
Dr. Al-Ali covers data on combined BET and JAK inhibition for patients with myelofibrosis presented at the 2023 ASH Meeting.
Worse survival in single-hit TP53 patients was mainly driven by bone marrow fibrosis.
SRSF2 and SF3B1 were the most common splicing mutations, followed by U2AF1 and ZRSR2.
Knowing who is at high risk in the near term could make it easier to conduct trials of anti-thrombotic therapies.
Developed by Protagonist Therapeutics, rusfertide is a novel injectable synthetic mimetic of the natural hormone hepcidin.
CALR and MPL mutations, which are associated with better outcomes, showed earlier mutation clearance than JAK2.
The retrospective study used data on 105 million U.S. patients with linked medical and prescription claims from 2007 to 2019.
Mass spectrometry–based proteomics were used to learn more about how MKs drive bone marrow fibrosis.
Prevalence of symptoms was assessed with the Myeloproliferative Neoplasm Symptom Assessment Form Total Symptom Score.
The retrospective study evaluated if pacritinib increased PLT counts in the phase III PERSIST-2 and phase II PAC203 studies.
Tasquinimod inhibited interaction of A9, a protein associated with poor prognosis.
DT2216 alone was effective, but exhibited a slightly greater cell viability reduction when combined with other therapies.
Responses in hemoglobin, white blood cell, and platelet were 75%, 82%, and 74%, respectively, at 24 weeks.
Multivariable modeling showed a notable reduction in the risk of GVHD or relapse in the ATG group.
Hemoglobin and platelets are correlated with OS, while mutations in ASXL1, EZH2, IDH1/2, U2AF1, and SRSF2 are not.
Rusfertide quickly induced hematocrit control and maintained improved levels over time in patients with polycythemia vera.
Subjectivity of morphological assessment and overlapping pathological features of subtypes can hamper accurate MPN diagnosis.
Bomedemstat is an oral lysine-specific demethylase-1 (LSD1) inhibitor clinically active in patients with MPNs.
The model could be helpful in understanding fibrotic progression in JAK2V617F mutation–positive MPNs.
The study aimed to address “a substantial unmet need for therapies that alter disease trajectory" in patients with MF.
Ruben Mesa, MD, discussed the results at the 65th ASH Annual Meeting and Exposition.
Researchers analyzed PV patients allocated to the ropeginterferon alfa-2b arm of the PROUD-PV/CONTINUATION-PV studies.
The use of combination therapies including XPO1 inhibitor selinexor is a potentially effective therapeutic strategy in MF.
This is just one finding from a longitudinal analysis of phase III data from the SIMPLIFY-1 and MOMENTUM trials.
Review more from the post-hoc time-dependent analysis of the phase III Simplify-1, Simplify-2, and MOMENTUM trials.
Led by Dr. Lindsay Rein, the goal of the phase I/II trial was to evaluate the safety and efficacy of TP-3654.
The multicenter trial studied genetic mutations across three cohorts.
CR rates were comparable in both treatment groups, but OS was superior in the ruxolitinib group.
Researchers also observed potential disease modification via rapid stabilization of platelets and stable hemoglobin levels.
There were no significant differences in response rates with pegylated interferon-alpha versus hydroxyurea in MPN treatment.
The trial is based on “compelling data” from arm III of the ongoing phase II MANIFEST study.
Primary myelofibrosis developed secondary neoplasms earlier and at higher rates versus other myeloproliferative neoplasms.
Essential thrombocythemia with CALR mutations had a higher risk of progression to myelofibrosis compared with JAK2 mutations.
A deep learning-based pathomics approach had potential in estimating bone marrow cellularity in patients with MPNs.
MPNs were not the primary cause of strokes in veterans, though researchers found they were strongly associated.
Patients with polycythemia vera developed secondary malignancies at a higher rate versus other myeloproliferative neoplasms.
82.5% of MF patients experienced rapid significant decreases in palpable spleen size after two months of ruxolitinib therapy.
The median duration of therapy is around three years in patients with intermediate or high-risk MF.
The analysis included 346 patients with CALR-mutated MF who were transplanted in 123 centers between 2005 and 2019.
Myelofibrosis Awareness Day is marked on September 20th each year.
Researchers presented updated data from the phase I XPORT-MF-034 trial at the Eleventh SOHO Annual Meeting.
The open-label ACE-536-MF-001 study included patient cohorts grouped by transfusion dependance and ruxolitinib therapy.
In PERSIST-2, treatment with pacritinib demonstrated a significant SVR benefit compared with the best available therapy, incl
Nearly all myelofibrosis patients are estimated to develop anemia over the course of the disease.
The need for phlebotomies substantially decreased in those who received ruxolitinib after hydroxyurea.
The combined average cost for the index hospitalization and two-year TE-related readmissions was $30,285.
Patients with MPNs faced higher direct and indirect costs and were significantly more likely to take disability leave.
The investigators sought to investigate the diagnostic landscape of polycythemia vera in LMICs.
Researchers presented data from the randomized withdrawal phase of the study at the SOHO Annual Meeting.
Ruxolitinib, an oral JAK1/JAK2 inhibitor, initially received FDA approval in 2011.
Researchers conducted the study because the mechanism by which pacritinib improves anemia has not been elucidated.
Researchers conducted an indirect comparison analysis of multiple clinical trials to address the question.
In June 2023, the manufacturer of the drug initiated XPORT-MF-034, a pivotal phase III clinical trial.
From Houston, Texas, to Beirut, Lebanon, the SOHO global community continues to grow thanks to its Ambassador Program.
At a median follow-up of 55 weeks, 90% of patients completed 24 weeks of treatment and 56% completed 48 weeks of treatment.
Initiation of ruxolitinib therapy within two years of diagnosis was associated with increased response rates in all patients.
Dr. Harrison discusses survey results from the LANDMARK survey in patients with polycythemia vera and their physicians.
Just under half (43%) of patients receiving ruxolitinib achieved a CR, while 26% achieved a CR on the best available therapy.
The trial included patients with PV who had at least three therapeutic phlebotomies in the 28 weeks prior to enrollment.
However, achieving a spleen volume reduction on the best available therapy was not linked with improved survival.
Dr. Mascarenhas and colleagues identified 11,371 patients with myelofibrosis, finding that 76.8% had concurrent anemia.
The median JAK2 mutant allele burden at baseline significantly differed among MPN subsets.
Dr. Kuykendall and colleagues presented their findings during the 2023 European Hematology Association Congress.
Dr. Sekeres also speaks about his time on the FDA ODAC and his latest book, titled "Drugs and the FDA."
Dr. Krecak presented the research during the 2023 EHA Congress.
Spleen volume reduction predicts OS in patients with myelofibrosis who are taking pacritinib.
A new study is exploring add-on treatment of CK0804 in patients with myelofibrosis and suboptimal response to ruxolitinib.
The phase II MANIFEST study suggested that pelabresib could be beneficial in myelofibrosis.
The study indirectly compared safety outcomes from phase II and phase III trials of momelotinib and fedratinib.
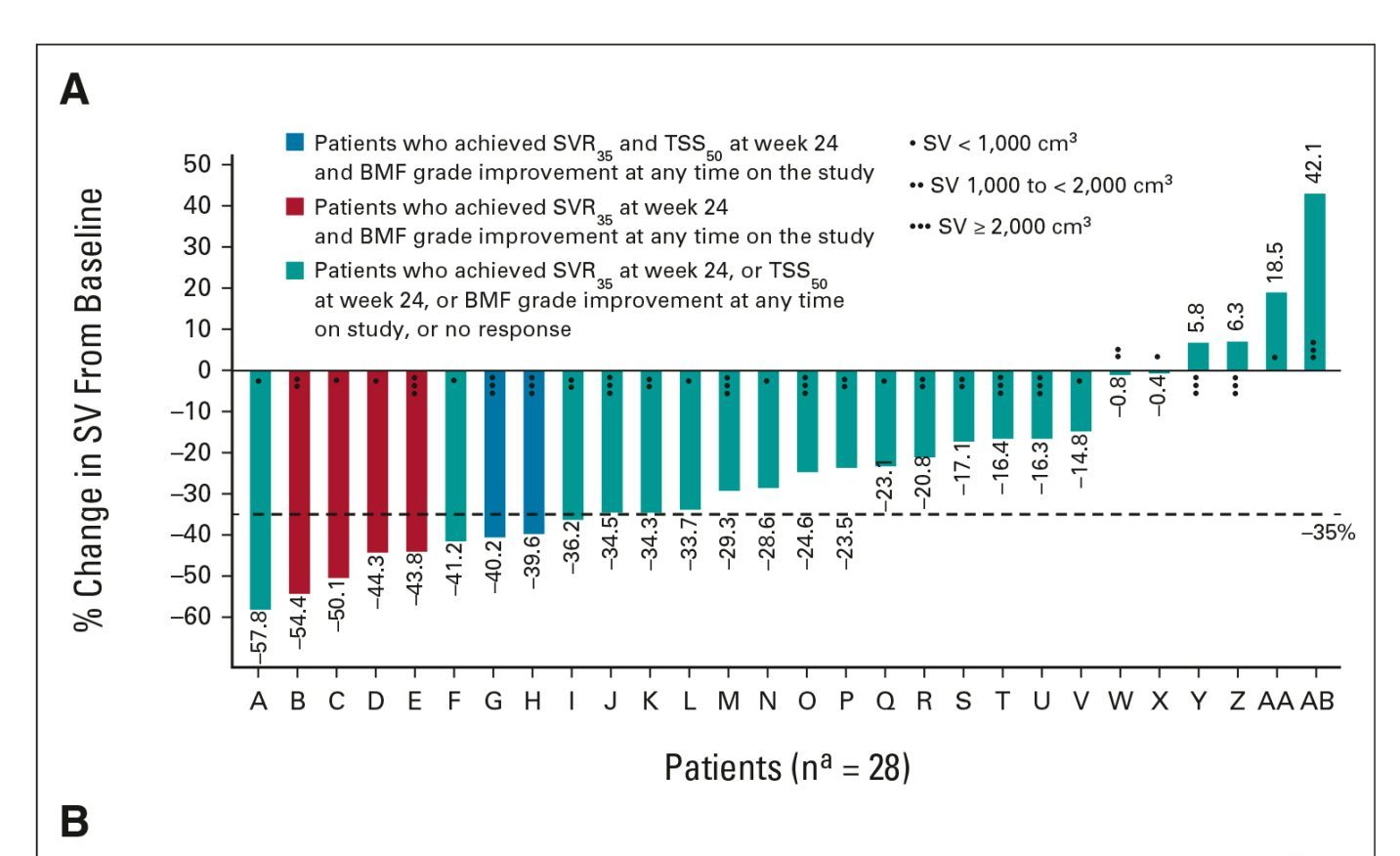
The median time to achieving the first spleen volume reduction of at least 35% from baseline was 12 weeks.
Prithviraj Bose, MD, and colleagues presented results of the study during the 2023 ASCO Annual Meeting.
The authors concluded that treatment with INCB057643 monotherapy was generally well tolerated.
The case-control study used information from the Veterans Affairs Informatics and Computing Infrastructure database.
The study is designed to evaluate the efficacy, safety, and tolerability of ropeginterferon alfa-2b-njft in adults with ET.
Researchers followed the patients for three years after their final dose of luspatercept.
Jakatinib may be a new effective treatment option for patients with myelofibrosis.
Pacritinib demonstrates consistent efficacy for spleen and symptom response in patients with MF regardless of blood counts.
Selinexor plus ruxolitinib was effective in certain subgroups of patients with myelofibrosis.
In patients with Afib, MPNs are linked to an increased risk of hospital readmissions for bleeding and arterial thrombosis.
The pooled analysis set included patients from both arms of the intent-to-treat populations in SIMPLIFY-1 and SIMPLIFY-2.
The trial will enroll certain patients who had an "inadequate response” to ruxolitinib alone.
The main aim of treatment is preventing thrombotic complications.
Dr. Tefferi discusses risk stratification systems for primary myelofibrosis, transplant considerations, and more.
They detected mutated TP53 in 49 (13%) patients, with 30 of those patients showing a multihit configuration.
The model was developed and validated using data from the CIBMTR and EBMT registries.
The study evaluated longitudinal symptom score changes to “complement the interpretation of the landmark symptom ...
Planning for a phase III trial of the combination is underway.
Jan Bewersdorf, MD, discusses a current ongoing phase I study investigating the combination of ruxolitinib and abemaciclib.
The FDA could not approve the application in its current form and identified additional requirements for approval.
Hematopoietic stem/progenitor cells from patients with myelofibrosis are “enriched” for a CXCL8/CXCR2 gene signature.
A reduction in spleen volume reduction of ≥35% at 24 weeks occurred in 68% of patients.
The study’s investigators are continuing to monitor overall survival and conduct ongoing patient follow-up.
Hematocrit control was “suboptimal” in more than half of patients with high-risk polycythemia vera.
The overall risk of death from primary myelofibrosis declined by more than 50% after the U.S. FDA approved ruxolitinib.
Ruben Mesa, MD, discusses his career in MPN treatment and research and notable abstracts at the 2022 ASH Annual Meeting.
Srdan Verstovsek, MD, discusses using machine learning to predict resistance to hydroxyurea therapy in polycythemia vera.
Patients with MPN have a high risk of death from cardiovascular causes.
Patients with MPN who are employed face a “high economic burden,” especially if they experience thrombotic events.
Statin use may reduce the risk of developing a myeloproliferative neoplasm (MPN), according to a recent study.
Chadi Nabhan, MD, MBA, FACP, interviews Srdan Verstovsek, MD, on highlights in MPN research at the 2022 ASH Annual Meeting.
Srdan Verstovsek, MD, discusses the phase III VERIFY trial of rusfertide in patients with polycythemia vera.
Mutation of CALR in patients with myelofibrosis may be associated with a more anemic phenotype at diagnosis.
John Mascarenhas, MD, discusses the results of the PACIFICA trial at the 2022 American Society of Hematology Annual Meeting.
Whole blood mutation allele frequency had only weak prognostic value in certain patients with polycythemia vera.
Several factors were independently associated with arterial thrombotic events in patients with myeloproliferative neoplasms.
Symptom-related quality of life is linked with the type of cytoreductive treatment used for MPNs.
TP53 and complex karyotype are very high-risk factors in patients with myelofibrosis undergoing HSCT.
The combination of ruxolitinib and pegylated IFNα2a showed significant reductions in spleen length.
BMS-986158 combined with ruxolitinib or fedratinib reduced spleen volume in patients with myelofibrosis.
Selinexor plus ruxolitinib demonstrated promising clinical activity in patients with treatment-naïve myelofibrosis.
The combination of interferon and ruxolitinib may be a treatment option for newly diagnosed polycythemia vera.
Momelotinib led to an increased likelihood of becoming transfusion-independent compared with danazol in myelofibrosis.
Long-term follow-up results of a phase II study of ruxolitinib in patients with MPNs were presented at the 2022 ASH Meeting.
New data support the use of ropeginterferon alfa-2b therapy in patients with low-risk or high-risk PV,
An analysis of patients with MF found that the combination of navitoclax and ruxolitinib reduced MF-associated splenomegaly.
IFNα2 treatment may help reduce the risk of arterial and venous thrombosis in patients with myeloproliferative neoplasms ...
Findings from a new study “underscore the limited therapeutic value of luspatercept” in anemia and myelofibrosis.
The revised classification includes many authors of the prior WHO edition but is not affiliated with the WHO.
The National Comprehensive Cancer Network publishes new MPN guidelines.
Researchers published new classifications for MPNs and acute leukemias.
Those with MF with high molecular and cytogenetic risk do not benefit from higher intensity conditioning before transplant.
Transfusion independence was associated with improved survival in studies of momelotinib in patients with myelofibrosis.
Adding navitoclax to ruxolitinib led to durable spleen volume reductions and improved total symptoms in myelofibrosis.
There are several risk factors for thrombosis and major bleeding in patients with myelofibrosis, according to a recent study.
A large analysis of real-world treatment outcomes in patients with myelofibrosis who were treated with ruxolitinib.
Momelotinib led to “superior” symptom and spleen responses and transfusion independence rates compared to danazol.
As TKIs have become available to patients, survival for those with CML has significantly improved but disparities.
VERIFY, a phase III trial of the hepcidin mimetic rusfertide (PTG-300), is now enrolling patients with PV.
IFN alpha-2 influences the regulation of several ECM genes involved in tissue remodeling.
A recent case study found evidence of an acquired in utero mutation in monozygotic twins who presented with CALR.
A new prognostic model identified risk factors for reduced survival in patients with myelofibrosis who received ruxolitinib.
The EXPAND study supports the use of ruxolitinib 10 mg twice daily in patients with MF.
A large real-world study assessed patients who were diagnosed with myeloproliferative neoplasms before age 25.
A retrospective study observed a three-year OS rate of 66.7% in primary MF patients and 55.6% in secondary MF patients...
The phase Ib clinical trial is being launched by Cellenkos in partnership with Incyte as part of their LIMBER initiative.
Study finds BM CH was present in nearly two-thirds of BPDCN patients and particularly prevalent in elderly patients.
Pankit J. Vachhani, MD, and Srdan Verstovsek, MD, debate the usefulness of variant allele frequency (VAF).
Long-term results from the RESPONSE-2 trial confirm efficacy of ruxolitinib for PV.
The REFINE trial observed clinically meaningful splenic responses with navitoclax plus ruxolitinib.
Tasquinimod is an oral immunomodulatory and antiangiogenic investigational treatment.
This phase II study enrolled 34 adult patients with intermediate- to high-risk myelofibrosis.
The NCCN now includes the oral JAK inhibitor pacritinib as a first-line treatment for certain myelofibrosis patients.
The FDA has granted accelerated approval to pacritinib for the treatment of adult patients with intermediate or high-risk.
In patients with high-risk essential thrombocythemia and polycythemia vera, both hydroxyurea and pegylated interferon-α.
Editor-in-Chief Sagar Lonial, MD, FACP, introduces the new publication from SOHO.
At SOHO 2021, Susan O'Brien, MD, and Hagop Kantarjian, MD, addressed trends in hematologic malignancies.
REVEAL is the largest prospective and contemporary cohort of patients with PV in the United States.
Researchers shared a new single-cell multi-omic analysis of the genetic, cellular, and molecular landscape of TP53-driven.
Experts discuss emerging therapeutic targets, the impact of recently approved agents, and incorporating them into practice.
Clonal hematopoiesis of indeterminate potential was associated with a reduced risk of Alzheimer's disease dementia.
A study evaluated factors associated with intense end-of-life care among patients younger than 40 with blood cancer.
A research team in Norway found that previous hematological malignancies were associated with a subsequent diagnosis.
Advertisement
Blood Cancers Today delivers the latest news, education, and information relevant to hematologic oncology patients and practices.
Sign up to receive Blood Cancers Today eNewsletters:
Advertisement





















































































































































 © 2025 Mashup Media, LLC, a Formedics Property. All Rights Reserved.
© 2025 Mashup Media, LLC, a Formedics Property. All Rights Reserved.