
Standard chemotherapy regimens, such as the combination of fludarabine, cyclophosphamide, and rituximab (FCR), previously held a long-standing recommendation as the standard of care for frontline chronic lymphocytic leukemia (CLL) therapy, especially for young and fit patients with CLL—loosely defined as patients under the age of 65 and with a Cumulative Illness Rating Scale ≤6 and creatinine clearance ≥70 mL/min. With the introduction of targeted kinase inhibitors, such as ibrutinib, acalabrutinib, and venetoclax, this recommendation has evolved over the last decade to incorporate the use of these agents in this population.
Here, Deborah Stephens, DO, describes the decision-making process for the treatment of newly diagnosed CLL, illustrated in 2 cases of young, fit patients with CLL. Dr. Stephens is Assistant Professor in the Division of Hematology and Hematologic Malignancies at the University of Utah and Physician Leader of the Hematology Clinical Trials Division at Huntsman Cancer Institute in Salt Lake City.
Presentation of Case #1
A 61-year-old man presented to the clinic with drenching night sweats and symptomatic splenomegaly. He was diagnosed with CLL and had no significant medical comorbidities. Cytogenetic and mutational analysis revealed the patient had mutated immunoglobulin variable heavy chain (IGHV) mutation and deletion of 13q (del13q).
Treatment Options for Case #1
FCR can lead to prolonged progression-free survival (PFS) in young patients (<65 years) with IGHV mutation, based on findings from the randomized, open-label, phase III CLL8 trial. Compared with fludarabine and cyclophosphamide, at 3 years after randomization, FCR improved rates of progression-free survival (PFS; 65% vs. 45%) and overall survival (OS; 87% vs. 83%).1
Subgroup analyses revealed that patients with IGHV-mutated CLL had the longest observed PFS and survival curves plateaued after 6 years. In an update from CLL8 published in 2016, there were no reported relapses beyond 10.4 years, but 5% of patients treated with FCR developed acute myeloid leukemia/myelodysplastic syndromes.2
Ibrutinib plus rituximab improved PFS over FCR in the randomized, phase III E1912 trial, with 3-year PFS rates of 89.4% versus 72.9%.3 Furthermore, in a subgroup analysis, ibrutinib-rituximab improved 3-year PFS over FCR in patients with unmutated IGHV (90.7% vs. 62.5%), but results were similar among patients with IGHV mutation (87.7% vs. 88.0%).
In the phase III ELEVATE-TN trial, the Bruton tyrosine kinase (BTK) inhibitor acalabrutinib with or without obinutuzumab prolonged PFS compared with chlorambucil plus obinutuzumab in older, treatment-naïve patients, with estimated 30-month PFS rates of 82% with acalabrutinib, 90% with acalabrutinib plus obinutuzumab, and 34% with obinutuzumab plus chlorambucil.4
Obinutuzumab was also evaluated in the CLL14 trial, which compared fixed-duration treatment with the BCL2 inhibitor venetoclax plus obinutuzumab with obinutuzumab plus chlorambucil in patients with previously untreated CLL and coexisting conditions.5 The 2-year PFS rate was significantly higher in the venetoclax group (88.2% vs. 64.1%). Researchers also noted that patients with mutated and unmutated IGHV experienced similar PFS benefits with venetoclax plus obinutuzumab.
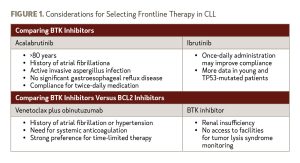
All these combinations are feasible for the patient in Case #1, but ibrutinib and acalabrutinib are given continuously, which may be less appealing compared to fixed duration therapy with venetoclax plus obinutuzumab for a young CLL patient.
Presentation of Case #2
A 59-year-old man presented with drenching night sweats and symptomatic splenomegaly and no significant medical comorbidities. After being diagnosed with CLL, the patient underwent mutational and cytogenetic testing. Analysis revealed unmutated IGHV and TP53 mutation.
Treatment Options for Case #2
Targeted agents should be used in young patients with CLL with high-risk disease secondary to improved PFS. Ibrutinib has the most data available, with the longest-term follow-up supporting its use in patients with TP53-mutated CLL. In a small study of patients with TP53-mutated CLL treated with ibrutinib, the agent produced a 6-year PFS rate of 61%.6 Acalabrutinib has been investigated for relapsed/refractory CLL, but data on its use as frontline treatment are limited. In a phase I/II study, single-agent acalabrutinib was associated with a 4-year PFS rate of 82%.7 Similarly, data for zanubrutinib are limited but promising, as seen in early results from arm C of the SEQUOIA study. The combination of venetoclax plus obinutuzumab has also shown promise, with 2-year PFS rates of 73% in the ongoing CLL14 study.8
Acalabrutinib, zanubrutinib, and venetoclax plus obinutuzumab have limited data, and longer follow-up is needed to define fully their utility in TP53-mutated patients. Given the similar results with available BTK inhibitors, choosing between these agents in the frontline setting should account for compliance and other patient characteristics, as outlined in Figure 1.
References
- Hallek M, Fischer K, Fingerle-Rowson G, et al. Addition of rituximab to fludarabine and cyclophosphamide in patients with chronic lymphocytic leukaemia: a randomised, open-label, phase 3 trial. Lancet. 2010;376(9747):1164-1161.
- Thompson PA, Tam CS, O’Brien SM, et al. Fludarabine, cyclophosphamide, and rituximab treatment achieves long-term disease-free survival in IGHV-mutated chronic lymphocytic leukemia. Blood. 2016;127(3):303-309.
- Shanafelt TD, Wang XV, Kay NE, et al. Ibrutinib-rituximab or chemoimmunotherapy for chronic lymphocytic leukemia. N Engl J Med. 2019;381(5):432-443.
- Sharman JP, Egyed M, Jurczak W, et al. Acalabrutinib with or without obinutuzumab versus chlorambucil and obinutuzmab for treatment-naive chronic lymphocytic leukaemia (ELEVATE TN): a randomised, controlled, phase 3 trial. Lancet. 2020;395(10232):1278-1291.
- Fischer K, Al-Sawaf O, Bahlo J, et al. Venetoclax and obinutuzumab in patients with CLL and coexisting conditions. N Engl J Med. 2019;380:2225-2236.
- Ahn IE, Tian X, Wiestner A. Ibrutinib for chronic lymphocytic leukemia with TP53 alterations. N Engl J Med. 2020;383(5):498-500.
- Byrd JC, Woyach JA, Furman RR, et al. Acalabrutinib in treatment-naïve chronic lymphocytic leukemia. Blood. 2021;137(24):3327-3338.
- Tedeschi A, Ferrant E, Flinn IW, et al. Zanubrutinib in combination with venetoclax for patients with treatment-naïve (TN) chronic lymphocytic leukemia (CLL) or small lymphocytic lymphoma (SLL) with del(17p): early results from Arm D of the SEQUOIA (BGB-3111-304) trial. Blood. 2021;138(Supplement 1):67.




 © 2025 Mashup Media, LLC, a Formedics Property. All Rights Reserved.
© 2025 Mashup Media, LLC, a Formedics Property. All Rights Reserved.