Take-aways:
- Maintenance therapy with eprenetapopt and azacitidine after allogeneic HSCT was well tolerated in patients with TP53-mutated AML and MDS.
- At a median follow-up of 14.5 months, the median RFS was 12.5 months, with a one-year RFS probability of 59.9%.
- The median OS was 20.6 months, with a one-year OS probability of 78.8% at a median follow-up of 17 months.
Maintenance therapy with a p53 reactivator plus azacitidine after allogeneic hematopoietic stem cell transplant (HSCT) led to “encouraging” outcomes and was well-tolerated in patients with TP53-mutated acute myeloid leukemia (AML) and myelodysplastic syndromes (MDS), according to a phase II, single-arm, multicenter, open-label clinical trial.
Asmita Mishra, MD, of the Moffitt Cancer Center, and colleagues conducted the clinical trial and published its results in the Journal of Clinical Oncology.
While the U.S. Food and Drug Administration granted Orphan Drug designation to eprenetapopt for treatment of AML in 2021, there are currently no approved targeted therapies for TP53 mutations, which are associated with poor outcomes in patients with AML and MDS, according to the authors.
“Allogeneic [HSCT] remains the only potential curative approach, but even after [allogeneic HSCT] the risk of relapse remains high and survival is poor,” Dr. Mishra and colleagues wrote.
Eprenetapopt plus azacitidine, the drug combination used in this trial, was previously tested in two parallel clinical trials that demonstrated the efficacy and safety of the treatment for patients with TP53-mutated AML or MDS.
Eprenetapopt is a first-in-class, small molecule p53 reactivator that functions as a prodrug and is converted into an active moiety, 2-methylene quinuclidine-3-one. It is believed to restore wild-type p53 function by stabilizing and reactivating p53 in TP53-mutant cells. The restoration of p53 function can selectively induce cell death in TP53-mutated cancer cells. Azacitidine is an antimetabolite antineoplastic agent.
Evaluating Eprenetapopt Plus Azacitidine in TP53-Mutated AML or MDS
The study included adults with AML or MDS who had a confirmed TP53 mutation, provided a pretransplant bone marrow biopsy, and were candidates for allogeneic HSCT. There were no pretransplant disease-related remission criteria and no restrictions on conditioning regimens, donor type, source, or graft-versus-host disease (GVHD) prophylaxis. There were no restrictions on therapeutic agents used prior to transplant. Patients who previously underwent allogeneic HSCT were excluded from the trial.
To be eligible for post-transplant maintenance therapy, patients needed to have a Karnofsky Performance Status score of at least 70, have <5% blasts for AML and ≤5% blasts for MDS, and be in cytogenetic remission with no morphological characteristics of disease in the bone marrow and no extramedullary disease. Patients needed to be at least 30 days but no more than 100 days from transplant and have confirmed engraftment to receive post-transplant maintenance therapy.
Exclusion criteria for post-transplant maintenance were use of an umbilical cord blood donor stem cell source; uncontrolled infection; use of hypomethylating agents, cytotoxic chemotherapeutic agents, or experimental agents for treatment of AML/MDS within 14 days of the first day of pretransplant screening or any time after; and use of experimental therapy for acute GVHD at any time after the transplant.
The researchers screened 84 patients pre-transplant and 55 patients post-transplant, 33 of whom enrolled and received eprenetapopt and azacitidine maintenance therapy. Of the 33 patients, 14 had AML and 19 had MDS. See TABLE 1 for the genetic characteristics of the patients.
The median patient age was 65 years (range, 40-74 years). Most patients (67%) received a reduced intensity conditioning regimen. The median time from transplant to maintenance therapy was 68 days.
Patients received eprenetapopt 3.7 g intravenously once daily on days one through four of each 28-day cycle. They received azacitidine 36 mg/m2 once daily intravenously or subcutaneously on days one through five in each 28-day cycle. Maintenance treatment continued for up to 12 cycles or until relapse, development of a comorbidity preventing treatment, unaccepted toxicity, patient withdrawal, investigator judgment, or another treatment for the underlying disease. The median number of eprenetapopt cycles was seven, with 13 patients (39%) completing all 12 cycles.
The primary endpoints of the study were relapse-free survival (RFS), as well as the incidence, severity, and relatedness of treatment-emergent adverse events and/or laboratory abnormalities.
Patient Responses to Eprenetapopt Plus Azacitidine
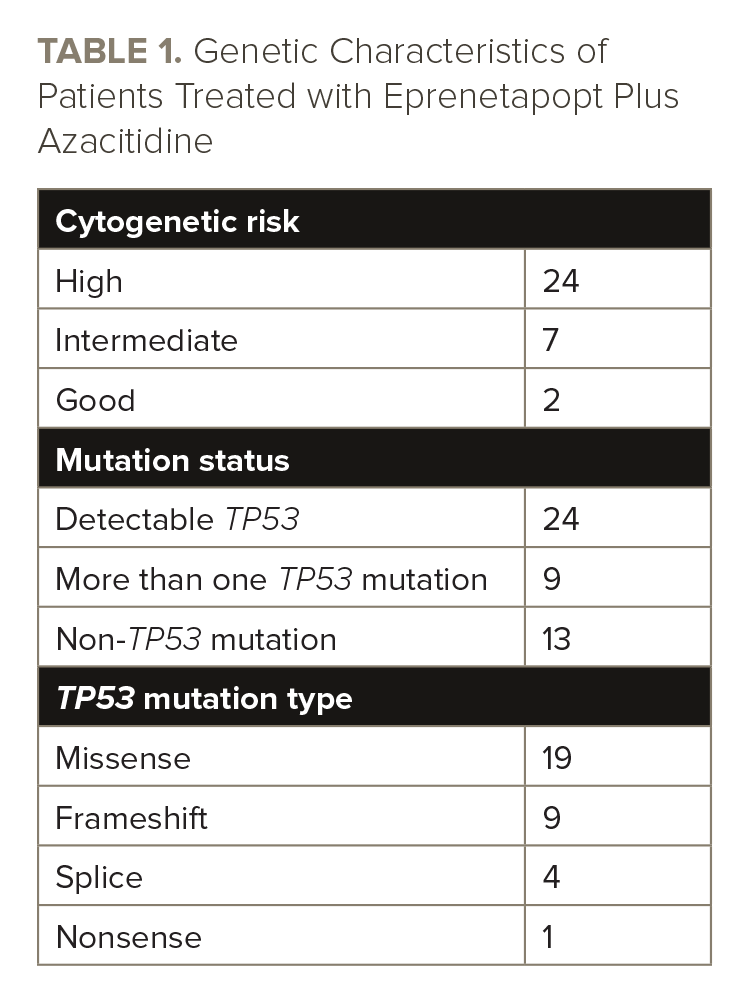
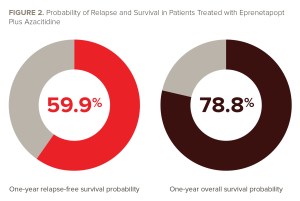
The median RFS was 12.5 months (95% CI, 9.6 to not estimable) at a median follow-up of 14.5 months. The one-year RFS probability was 59.9% (95% CI, 41.0-74.4). The median overall survival (OS) was 20.6 months, with a one-year OS probability of 78.8% at a median follow-up of 17 months (95% CI, 60.6-89.3). The one-year cumulative incidence of relapse rate was 38.3% (95% CI, 23.7-57.6), and the one-year non-relapse mortality rate was 3.8% (95% CI, 0.6-24.3). The results are summarized in FIGURES 1 and 2.
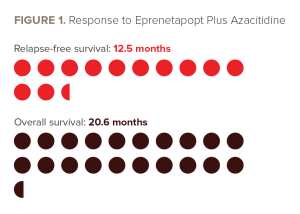
The investigators conducted exploratory analyses using the MyMRD Gene Panel Assay on pretransplant samples for all patients with available samples (n=29). Researchers additionally analyzed on-treatment samples (n=20) for patients treated at the Moffitt Cancer Center and compared them with the patients’ pretransplant samples. Most patients (82%) had detectable mutant TP53 in their pretransplant sample.
There was “no apparent relationship” between dominant baseline TP53 variant allele frequency (VAF) and RFS, nor OS, according to Dr. Mishra and colleagues. All four patients who completed fewer than 12 treatment cycles relapsed and had samples available had an increase in mutant TP53 coincident with relapse.
“Despite the established association between [mutant] TP53 VAF, the number of TP53 mutations, and adverse prognosis in patients with complex karyotype MDS and AML, there was no significant association between the treatment duration and pre-[transplant]–dominant [mutant] TP53 allele VAF or the number of pretransplant non-TP53 mutations,” Dr. Mishra and colleagues wrote.
The most common grade ≥3 adverse events were decreased platelet count and decreased white blood cell count, which were each reported in 36% of patients. Other common events were decreased neutrophil count (27% of patients), anemia (27%), thrombocytopenia (12%), and hypertension (12%). The most common serious adverse events were pyrexia (12%), febrile neutropenia (6%), and dyspnea (6%). The researchers reported GVHD in 39% of patients. The 30-day mortality rate from the first dose was 0%, while the 60-day mortality rate from the first dose was 6%.
“In conclusion, post-[transplant] maintenance therapy with eprenetapopt plus azacitidine in this high-risk population of patients with [mutant] TP53 AML or MDS led to favorable survival outcomes,” Dr. Mishra and colleagues wrote. “These data support future exploration of this maintenance strategy in a phase III, randomized, controlled, double-blind study of eprenetapopt plus azacitidine versus placebo plus azacitidine in patients with [mutant] TP53 myeloid malignancies.”
This study was supported by Aprea Therapeutics.
Reference
Mishra A, Tamari R, DeZern AE, et al. Eprenetapopt plus azacitidine after allogeneic hematopoietic stem-cell transplantation for TP53-mutant acute myeloid leukemia and myelodysplastic syndromes. J Clin Oncol. 2022. doi:10.1200/JCO.22.00181






 © 2025 Mashup Media, LLC, a Formedics Property. All Rights Reserved.
© 2025 Mashup Media, LLC, a Formedics Property. All Rights Reserved.