Take-aways:
- The addition of navitoclax to ruxolitinib led to durable reductions in spleen volume as well as improvements in overall symptoms in patients with myelofibrosis that was relapsed or refractory to ruxolitinib monotherapy.
- The safety profile of this combination was similar to that of ruxolitinib alone, with the exception of a higher rate of thrombocytopenia.
- Further studies evaluating this treatment regimen, including allelic burden and biomarker modification analyses, are ongoing.
In patients with persistent or progressive myelofibrosis, adding the BCL-XL/BCL-2 inhibitor navitoclax to therapy with the JAK1/2 inhibitor ruxolitinib led to durable spleen volume reduction as well as improvements in overall symptoms, hemoglobin response, and bone marrow fibrosis (BMF). This is according to research findings published in the Journal of Clinical Oncology.
“The findings suggest disease-modifying activity in a population with limited therapeutic options after ruxolitinib unresponsiveness or resistance,” the authors, led by Claire N. Harrison, MD, of Guy’s and St. Thomas’ Hospital in London, wrote.
This phase II study enrolled 34 adult patients with intermediate- to high-risk myelofibrosis who experienced disease progression or suboptimal response following ruxolitinib monotherapy at a stable dose of at least 10 mg twice daily. Patients were mostly male (n, 23; 68%), their median age was 68 years old (range, 42-86), and they had previously been exposed to ruxolitinib for a median of 82 weeks (range, 19-308). Among the 33 patients who were screened for mutations at enrollment, 29 (79%) had JAK2V617F mutations and 7 (21%) had CALR mutations.
The primary endpoint of the study was a spleen volume reduction of at least 35% (SVR35) from baseline at week 24. Safety, hemoglobin improvement, change in BMF, and a greater than 50% reduction in total symptom score (TSS) from baseline were also evaluated as secondary endpoints. Patients also followed for survival every 6 months and will be followed for up to 5 years after treatment discontinuation, the researchers explained.
As of data cutoff on August 30, 2020, 24 patients (71%) remained on study, while the remainder had either died (n, 5), withdrawn consent from follow-up (n, 2), discontinued to undergo stem cell transplantation (n, 2), or discontinued because of progressive disease (n, 1).
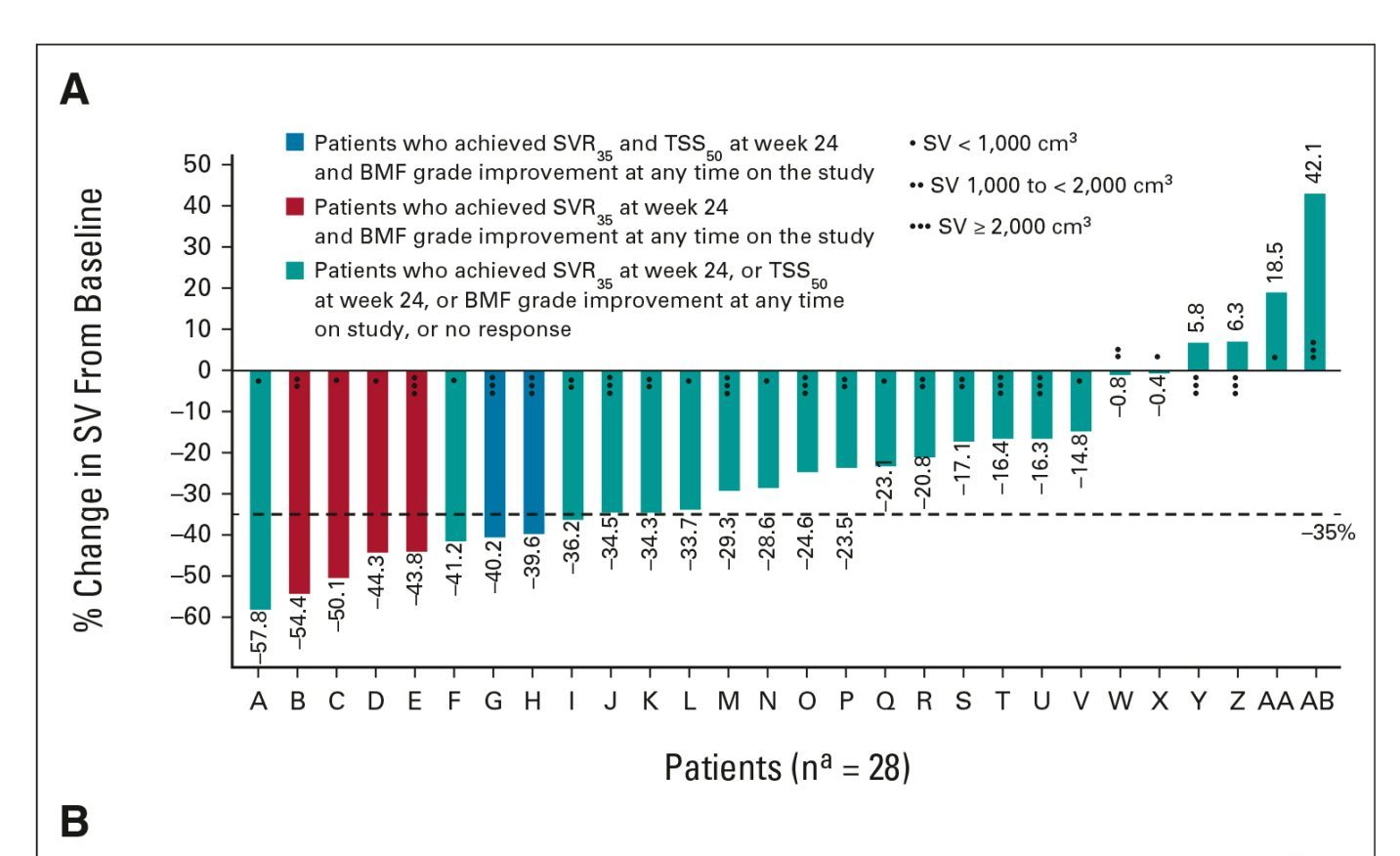
At week 24, 9 patients had achieved the primary endpoint of SVR35, with a median spleen volume of 1,069 cm3, compared with 1,695 cm3 at baseline. A total of 14 patients achieved the primary endpoint at any time on study, as late as week 72. Six patients achieved this response at week 12 and 8 at week 48. Overall, the median duration of SVR35 was 13.8 months, regardless of the point when it was achieved during the study.
Among the 20 patients who were evaluable for TSS at week 24, 6 (30%) reported a greater than 50% reduction in symptoms. A total of 12 patients achieved this endpoint at any time during the study period, the authors added.
Regarding BMF assessment, 33 patients had available data. Eleven patients (33%) experienced an improvement in BMF by at least 1 grade from baseline at any time on study. The remaining 22 had either equal or worsened BMF from baseline. Details about responses are presented in the Figures.
Additionally, median overall survival (OS) was not reached at a median follow-up of 21.6 months. “This suggests that the addition of navitoclax to ruxolitinib may result in increased OS compared with conventional (e.g., danazol, hydroxyurea, etc.) or targeted (e.g., investigational JAK2 or non-JAK2 inhibitors, etc.) therapies received after ruxolitinib discontinuation,” the authors noted. They added that the median OS for both conventional and targeted therapies in this setting is up to 14 months.
Most patients (n, 30; 88%) experienced grade ≥3 adverse events (AEs). The most common grade ≥3 AEs included thrombocytopenia without clinically significant bleeding (n, 19; 56%), anemia (n, 11; 32%), and pneumonia (n, 4; 12%). Serious AEs were reported in 15 patients (44%) and the most common were pneumonia (n, 4; 12%) and splenic infarction (n, 2; 6%). A total of five patients died during the study from pneumonia with contributing acute kidney injury and pulmonary edema (n, 1), COVID-19 (n, 1), disease progression and respiratory failure (n, 1), pneumonia and hematoma after a fall (n, 1), and respiratory failure secondary to infection (n, 1).
“Further investigation is underway to qualify the potential for disease modification,” the authors wrote. The combination is currently being evaluated for patients with myelofibrosis in two global phase III clinical trials, TRANSFORM-1 (NCT04472598) and TRANSFORM-2 (NCT04468984).
Disclosures: This research was supported by AbbVie. Study authors report relationships with AbbVie, the manufacturer of navitoclax.
Reference
Harrison CN, Garcia JS, Somervaille TCP, et al. Addition of navitoclax to ongoing ruxolitinib therapy for patients with myelofibrosis with progression or suboptimal response: phase II safety and efficacy. J Clin Oncol. 2022 Feb 18; doi: 10.1200/JCO.21.02188.






 © 2025 Mashup Media, LLC, a Formedics Property. All Rights Reserved.
© 2025 Mashup Media, LLC, a Formedics Property. All Rights Reserved.