The addition of pelabresib, an investigational bromodomain and extraterminal domain (BET) inhibitor, to ruxolitinib therapy can lead to significant response in patients with myelofibrosis. This response was observed in the phase III MANIFEST-2 study, which was presented at the Society of Hematologic Oncology 2024 Annual Meeting in Houston, Texas.
“Pelabresib may enhance clinical responses to ruxolitinib by further reducing [Janus kinase 2 (JAK2) variant allele fraction (VAF)] levels and decreasing NF-kB–regulated cytokines. The latter was associated with clinical responses,” the authors wrote regarding their findings.
In this study, 46 patients received an oral pelabresib plus ruxolitinib regimen, while 37 received placebo plus ruxolitinib. The investigators performed mutational analyses and evaluated biomarker results between the two groups using peripheral blood samples.
The percentage of patients who had achieved a 20% or greater JAK2 VAF reduction at week 48 of the study was 41.3% in the pelabresib plus ruxolitinib group, compared with 37.8% in the placebo plus ruxolitinib group (P<.001). This reduction was associated with patients having a reduction in spleen volume from baseline of 35% or greater at week 24.
Reduction in NF-kB–regulated proinflammatory cytokine levels at week 24 was greater among patients who received the pelabresib plus ruxolitinib regimen (-33.9%) than among those who received placebo plus ruxolitinib (-20.8%) (P<.001).
These reduced levels were also associated with patients having a spleen volume reduction of 35% or greater from baseline (P<.001) and more frequent reporting of a 50% or greater reduction in total symptom score from baseline at week 24 (P=.005).
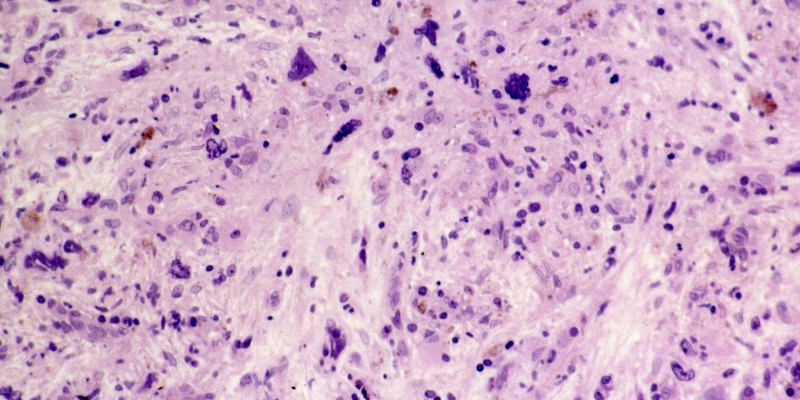
At week 24 of the study, the pelabresib plus ruxolitinib group had shown greater reductions than the placebo plus ruxolitinib group in both reticulin fiber density and megakaryocytes at -5.2 arbitrary units versus -1.0 arbitrary units (P<.001) and -54.5 cells/mm2 versus -27.4 cells/mm2 (P=.012), respectively.
CD71+ erythrocyte progenitor cell (EPC) proportions had an increase from baseline of 11.4% in the pelabresib plus ruxolitinib group and a decrease from baseline of 9.8% in the placebo plus ruxolitinib group (P=.019). Patients who did not require red blood cell transfusions saw greater increases in EPCs at week 24.
“Pelabresib plus ruxolitinib improved the bone marrow microenvironment. EPC increase was associated with anemia amelioration,” the investigators noted about these results.
The development of pelabresib is partially funded by the Leukemia and Lymphoma Society.
Reference
Mascarenhas J, Rampal RK, Vannucchi AM, et al. Pelabresib plus ruxolitinib combination therapy in JAK inhibitor-naïve patients with myelofibrosis in the phase 3 MANIFEST-2 study: Preliminary evidence of bone marrow recovery. Abstract #MPN-136. Presented at the Society of Hematologic Oncology 2024 Annual Meeting; September 4-7, 2024; Houston, Texas.






 © 2025 Mashup Media, LLC, a Formedics Property. All Rights Reserved.
© 2025 Mashup Media, LLC, a Formedics Property. All Rights Reserved.