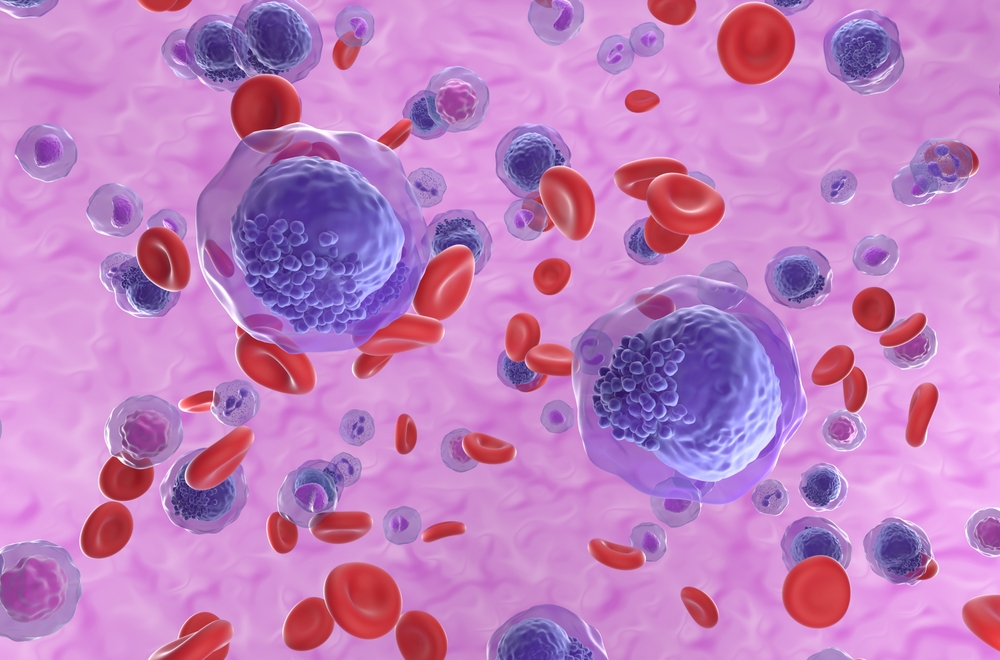
The U.S. Food and Drug Administration (FDA) has granted Orphan Drug Designation to DSP-5336, an investigational small-molecule inhibitor against the binding of menin and mixed-lineage leukemia (MLL) protein, for the treatment of acute myeloid leukemia (AML).
Menin is a scaffold nuclear protein that plays various key roles in biological pathways, including cell growth regulation, cell cycle control, genomic stability, bone development, and hematopoiesis. DSP-5336 is a small molecule that blocks the MLL-menin partnership, which inhibits normal differentiation and promotes leukemia growth.
DSP-5336 is currently being evaluated in a phase I/II clinical trial in the United States and Japan to evaluate its safety and efficacy in patients with relapsed or refractory AML and acute lymphoblastic leukemia with or without MLL rearrangement or NPM1 mutation.
Source: PR Newswire via Sumitomo Pharma Oncology, Inc, August 2022




 © 2025 Mashup Media, LLC, a Formedics Property. All Rights Reserved.
© 2025 Mashup Media, LLC, a Formedics Property. All Rights Reserved.