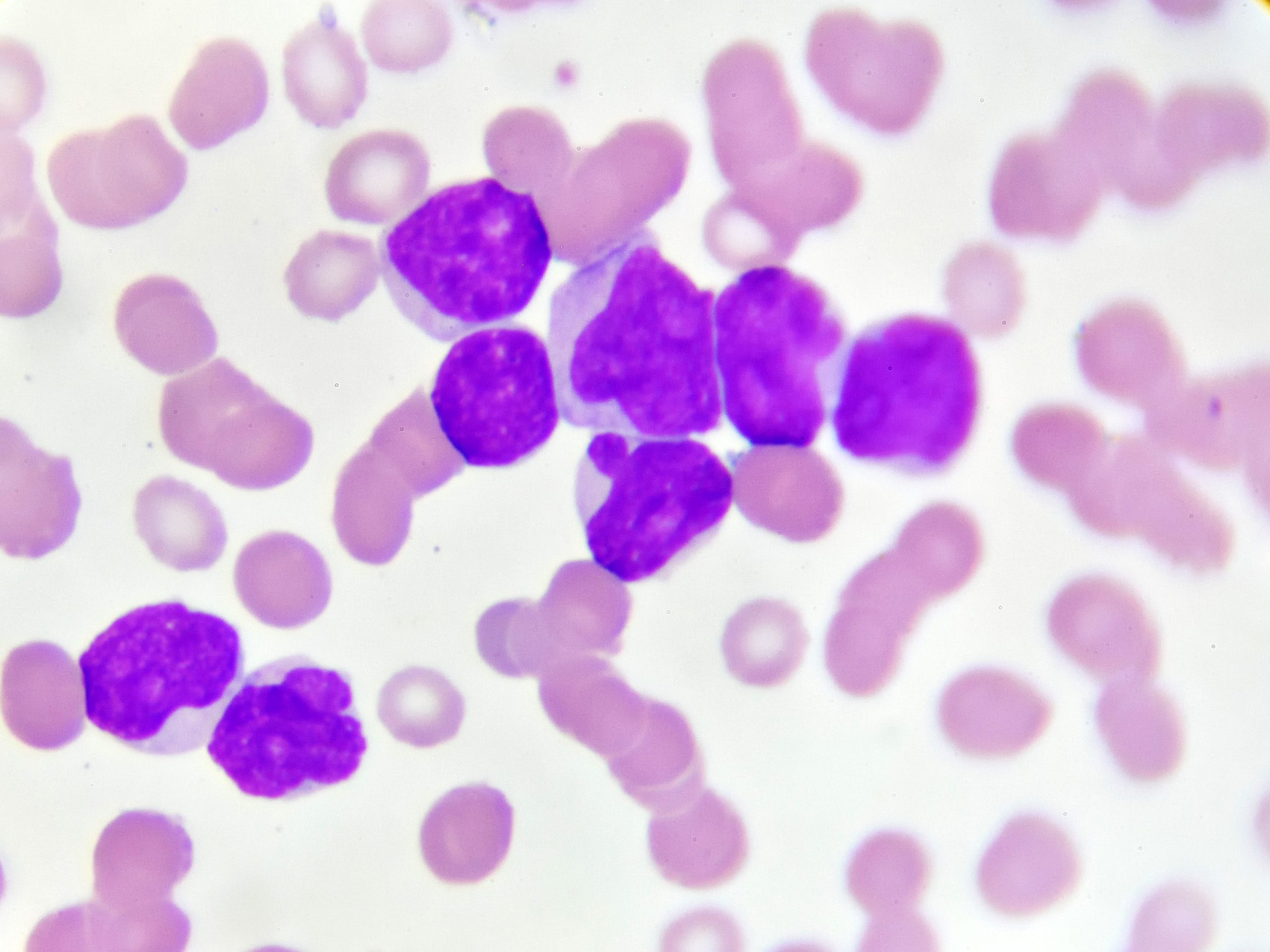
The U.S. Food and Drug Administration (FDA) has granted orphan drug designation to NKX101 (allogeneic CAR NK cells targeting NKG2D ligands) for the treatment of acute myeloid leukemia (AML).
NKX101 is an immunotherapy that uses natural killer cells from the peripheral blood of healthy donors, engineered with membrane-bound IL-15 (a cytokine for activating NK cell growth) and a chimeric antigen receptor targeting NKG2D ligands on cancer cells.
Currently, a phase I clinical trial is evaluating NKX101 in adult patients with relapsed/refractory AML or intermediate-, high-, or very high–risk myelodysplastic syndrome. Initial data will be presented in the first half of 2022, according to a press release by the treatment’s manufacturer, Nkarta.
Source: Nkarta Therapeutics press release, December 16, 2021.




 © 2025 Mashup Media, LLC, a Formedics Property. All Rights Reserved.
© 2025 Mashup Media, LLC, a Formedics Property. All Rights Reserved.