
The major take-aways of this research include the following: (1) Revumenib was associated with “promising” results in patients with AML who had a KMT2A rearrangement or NPM1 mutation; (2) No responses occurred in the patients who did not have a KMT2A rearrangement or NPM1 mutation; (3) The first-in-human study of revumenib demonstrated that inhibition is a therapeutic strategy for AML with KMT2A rearrangement or NPM1 mutation.
A first-in-human, phase I clinical trial showed the menin inhibitor revumenib was associated with “promising antileukemic activity leading to deep and sustained remission” in certain patients with relapsed or refractory acute leukemia.
Ghayas Issa, MD, of the University of Texas MD Anderson Cancer Center, and colleagues conducted the trial evaluating revumenib, which is a potent, selective oral inhibitor of interaction between menin and the KMT2A protein. The interaction between menin and KMT2A, an epigenetic regulator, is a “dependence” in acute leukemias caused by rearrangement of KMT2A or a mutation in the NPM1 gene, according to the study’s authors.
KMT2A rearrangements, which can occur in approximately 10% of acute leukemias, have an “adverse prognosis,” and NPM1 mutations, which occur in up to 30% of cases, are the “most common genetic alteration in acute myeloid leukemia,” Dr. Issa and colleagues wrote. However, there are “no targeted therapies specifically approved” for acute leukemia with KMT2A rearrangement or mutated NPM1.
Phase I Trial Design and Patient Characteristics
The phase I trial tested revumenib in 60 adults and eight children with relapsed or refractory acute leukemia. Adults had a median age of 50.5 years, while children had a median age of 2.5 years.
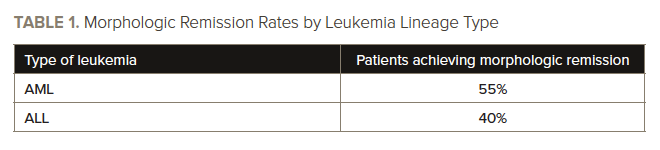
Most patients (82%) had acute myeloid leukemia (AML), 16% had acute lymphoblastic leukemia (ALL), and 2% had mixed-phenotype acute leukemia. Approximately two-thirds (68%) of patients had KMT2A rearrangements, 21% had mutated NPM1, and 12% had neither KMT2A rearrangements nor NPM1 mutations.
The patients were “heavily pretreated” and had a median of four previous lines of therapy, with 46% relapsing after receiving an allogeneic hematopoietic stem cell transplantation (HSCT), according to the study’s authors.
Responses to Revumenib Treatment
Complete remission (CR) or CR with partial hematologic recovery occurred in 30% of the 60 patients who were evaluable. Of those patients, 78% had undetectable measurable residual disease, as assessed by multiparameter flow cytometry. The median time to CR or CR with partial hematologic recovery was 1.9 months.
“Although imaging assessment was not mandated on study, responses were notably seen in both bone marrow and extramedullary sites in two of six evaluable patients with extramedullary leukemia at enrollment,” Dr. Issa and colleagues wrote.
In patients who achieved CR or CR with partial hematologic recovery, the median duration of response was 9.1 months (95% CI, 2.7 to not reached) at a median follow-up of 11.9 months. The median overall survival was seven months (95% CI, 4.3-11.6) in the efficacy population, regardless of remission status, at a median follow-up of 14.3 months (95% CI, 10.6-16.7).
Furthermore, 12 patients received allogeneic HSCT as consolidation following their response to revumenib. Of those patients, nine were in remission at the time of data cutoff, seven of whom were in remission for more than six months.
Most patients (84%) who had KMT2A rearrangement and achieved morphologic remission after the first cycle of revumenib “retained the detectable fusions causing KMT2A [rearrangement] during concomitant cytogenetic analyses,” the study’s authors wrote, noting that “most patients later had clearance of these KMT2A rearrangements with subsequent cycles of therapy.”
Patients with KMT2A rearrangement who achieved morphologic clearance of myeloblasts had a 64% complete cytogenetic response rate, with a median time to achieving cytogenetic CR of 1.9 months (range, 0.9-2.8 months).
No responses occurred in the eight patients who did not have a KMT2A rearrangement or NPM1 mutation, which was “consistent with the preclinical hypothesis regarding the efficacy of menin inhibition in patients with NPM1 mutations or KMT2A [rearrangement],” Dr. Issa and colleagues wrote.
The authors also conducted an exploratory descriptive analysis to evaluate responses by leukemia lineage and patient age, finding morphologic remission occurred in four of the eight (50%) children and in 28 of the 52 (54%) adults. See TABLE 1 for a summary of responses by leukemia lineage.
Menin Inhibition Established as a Therapeutic Strategy
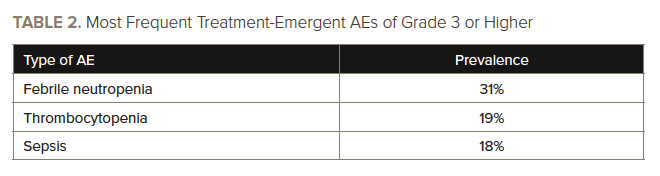
Nearly all (99%) of the 68 patients who received treatment had an adverse event (AE) during treatment, with a treatment-related AE of any grade occurring in 78% of patients.
However, the therapy was associated with a “low frequency” of grade 3 or higher treatment-related AEs, Dr. Issa and colleagues wrote. See TABLE 2 for information on treatment-emergent AEs of grade 3 or higher. Asymptomatic prolongation of the QT interval on electrocardiography was the only dose-limiting toxicity.
While treating patients who have relapsed or refractory acute leukemia with KMT2A rearrangement is “challenging,” with low rates of CR with incomplete count recovery after two lines of therapy, the menin inhibitor has the “potential to address these unmet needs,” the authors wrote.
The data from the first-in-human study “establish menin inhibition as a therapeutic strategy for susceptible” acute leukemia subtypes, according to the study’s authors.
“In children and adults with highly refractory acute leukemia with KMT2A [rearrangement] or NPM1 mutation, menin inhibition with revumenib monotherapy was associated with promising antileukemic activity leading to deep and sustained remission,” Dr. Issa and colleagues concluded.
This study was funded by Syndax Pharmaceuticals.
Reference
Issa GC, Aldoss I, DiPersio J, et al. The menin inhibitor revumenib in KMT2A-rearranged or
NPM1-mutant leukaemia. Nature. 2023. doi:10.1038/s41586-023-05812-3






 © 2025 Mashup Media, LLC, a Formedics Property. All Rights Reserved.
© 2025 Mashup Media, LLC, a Formedics Property. All Rights Reserved.