
Take-aways
- In patients with newly diagnosed AML, 94% experienced a complete remission or complete remission with incomplete hematologic recovery.
- Three-quarters of evaluable patients experienced complete cytogenetic complete response, and more than half achieved measurable residual disease negativity.
- The most frequent treatment-emergent nonhematologic adverse events included hypokalemia, hypophosphatemia, and hyperbilirubinemia. Anemia was manageable, with no treatment interruptions or discontinuations.
According to results presented at the 2021 American Society of Hematology Annual Meeting, more than 90% of older and high-risk patients with newly diagnosed acute myeloid leukemia (AML) had a complete remission (CR) following treatment with magrolimab and venetoclax plus azacitidine. The findings from the phase I/II study of the magrolimab triplet combination were presented by Naval Daver, MD, of The University of Texas MD Anderson Cancer Center in Houston.
Per the study investigators, most patients with AML experience relapse despite high response rates to front-line treatment with azacitidine and venetoclax. In the phase I portion of this study, Dr. Daver and researchers assessed whether the addition of magrolimab to azacitidine and venetoclax in adults with AML could improve disease outcomes.
Overall, the study included 38 participants, all of whom had AML with an Eastern Cooperative Oncology Group performance scores of 0 to 2, white blood cell counts less than 15 × 109/L, and adequate organ function. The initial phase Ib trial enrolled 6 patients with relapsed/refractory AML. There were no dose-limiting toxicities at the end of the phase Ib portion of the study, which suggested a recommended phase II dose of magrolimab 1 mg/kg on days 1 and 4 of cycle 1, 15 mg/kg on day 8 of cycle 1, and 30 mg/kg on day 11 of cycle 1.
After establishing the recommended phase II dose, an expanded protocol allowed for the enrollment of 11 more newly diagnosed patients and 21 patients with relapsed/refractory AML (8 venetoclax-naïve and 13 venetoclax-exposed). Patients in the front-line cohort were 75 years or older, had comorbidities that made them ineligible for intensive therapy, or had adverse-risk karyotype and/or TP53 mutation regardless of age/fitness.
The median age of the newly diagnosed patients was 70 years (range, 33-84). Most patients had high-risk features, including 82% who were categorized as European LeukemiaNet adverse-risk and 47% who had TP53 mutation.
Each 4-week treatment cycle consisted of azacitidine 75 mg/m2 (days 1-7) and venetoclax 400 mg (days 1-28). Magrolimab was dosed on days 1, 4, 8, 11, 15, and 22 of cycle 1. In addition, magrolimab was dosed weekly in cycle 2, then every 2 weeks in cycle 3 onward.
In the phase II portion of the study, the investigators focused on assessing the triplet combination’s safety and maximum tolerated dose (MTD). Secondary objectives were the rates of complete remission (CR)/CR with incomplete hematologic recovery (CRi), duration of response (DOR), and overall survival (OS).
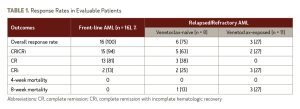
Of the 16 patients with newly diagnosed AML who were evaluable for response, the CR/CRi rate was 94%, and there were no patient deaths reported at the 8-week timepoint. All patients had a response after the first cycle of treatment. Among the patients with CR/CRi, the median time to absolute neutrophil count recovery was 28 days, and the median time to platelet recovery was 24 days.
Three-quarters of evaluable patients experienced complete cytogenetic CR (n, 9/12) and more than half achieved measurable residual disease negativity (n, 7/13). The authors reported that, after a median follow-up of 3.4 months, 1 of the 17 patients with newly diagnosed AML died from disease relapse and 1 patient had relapsed disease and is on alternate therapy. The remaining 15 patients continued on study protocol.
Among the entire cohort of 38 patients, the 8-week mortality rate was 9.7%, and all deaths occurred in patients with relapsed/refractory disease. Notably, among the patients with relapsed/refractory AML, prior venetoclax exposure appeared to be associated with lower CR/CRi rates (27% for venetoclax-exposed vs. 63% for venetoclax-naïve). See Table 1 for more details on outcomes.
The most frequent treatment-emergent non-hematologic adverse events (AEs) included hypokalemia (58%), hypophosphatemia (55%), hyperbilirubinemia (53%), hyponatremia (53%), and sinus tachycardia (47%). Grade 3/4 AEs included pneumonia (32%), febrile neutropenia (32%), hyperbilirubinemia (11%), elevated ALT (11%), and skin infection (11%).
Disclosures: This research was supported by the National Cancer Institute and MD Anderson Cancer Center. Study authors report financial relationships with Gilead Sciences, the manufacturer of magrolimab.
Reference
Daver N, Konopleva M, Maiti A, et al. Phase I/II study of azacitidine (AZA) with venetoclax (VEN) and magrolimab (magro) in patients (pts) with newly diagnosed older/unfit or high-risk acute myeloid leukemia (AML) and relapsed/refractory (R/R) AML. Abstract #371. Presented at the 2021 American Society of Hematology Annual Meeting, December 12, 2021.




 © 2025 Mashup Media, LLC, a Formedics Property. All Rights Reserved.
© 2025 Mashup Media, LLC, a Formedics Property. All Rights Reserved.