
Experts discuss the current research in the field of hematologic malignancies, from emerging therapeutic targets to the impact of recently approved agents, and how hematologists/oncologists can incorporate these findings into practice.
The following material is reproduced from “SOHO State of the Art Updates and Next Questions: Novel Therapies in Development for Myelofibrosis,” published in the Oct. 12, 2021, issue of Clinical Lymphoma, Myeloma & Leukemia. The article was written by Helen T. Chifotides, PhD; Prithviraj Bose, MD; Lucia Masarova, MD; Naveen Pemmaraju, MD; and Srdan Verstovsek, MD, PhD.
Novel Therapies in Development for Myelofibrosis
The regulatory approval of ruxolitinib for intermediate- and high-risk myelofibrosis (MF) heralded the era of Janus kinase (JAK) inhibition in the therapy of MF. Ruxolitinib revolutionized MF management and became the centerpiece of treatment as the sole agent for nearly a decade until the second oral JAK2 inhibitor fedratinib was approved in the United States in 2019.
The 2 registered JAK2 inhibitors elicit marked clinical benefits in 2 hallmarks of the disease, namely splenomegaly and constitutional symptoms. Notwithstanding the tremendous clinical benefits and unprecedented improvement in patient quality of life that JAK inhibitors have conferred, certain limitations and major unmet needs in specific subgroups of patients remain.
A plethora of investigational agents are being developed in earnest, and the MPN field has entered a new, burgeoning era with an array of highly promising medications on the horizon (Table 1). Novel regimens include less myelosuppressive JAK inhibitors, agents targeting alternate biological mechanisms beyond the JAK/STAT pathway, and ruxolitinib-based combinations that may enhance or complement the efficacy of ruxolitinib.
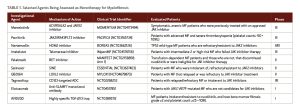
Investigational JAK Inhibitors
Despite the remarkable success of the approved JAK inhibitors in improving quality of life and managing splenomegaly and constitutional symptoms, the critical challenges of anemic and severely thrombocytopenic MF patients spurred the clinical development of the investigational JAK inhibitors momelotinib and pacritinib.
Momelotinib
Momelotinib is a JAK1/2 inhibitor with the unique ability among JAK inhibitors to also inhibit the type 1 kinase activin A receptor or activin receptor-like kinase-2 (ACVR1/ALK2), which is a transmembrane protein on hepatocytes expressing the master iron regulator, hepcidin.
In the randomized SIMPLIFY-1 study of JAK inhibitor–naïve patients and the randomized SIMPLIFY-2 study, momelotinib led to higher rates of transfusion-independence than ruxolitinib or best available therapy (BAT). Momelotinib also has demonstrated significant clinical efficacy with respect to spleen volume reduction (SVR), which was not statistically different from that of ruxolitinib and fedratinib according to a recent systematic review and network meta-analysis of 7 randomized phase III trials.
The efficacy of momelotinib in improving iron-restricted anemia, besides splenomegaly and constitutional symptoms—the 3 cardinal features of MF—is currently being further evaluated in the international phase III MOMENTUM trial, which is comparing momelotinib to danazol in JAK inhibitor–exposed patients.
Pacritinib
Pacritinib is a relatively nonmyelosuppressive JAK2/IRAK1/FLT3 inhibitor that was assessed in MF patients in 2 phase III clinical trials, PERSIST-1 and PERSIST-2. In PERSIST-1, JAK inhibitor–naïve patients with MF were randomized to pacritinib or BAT (excluding ruxolitinib). In PERSIST-2, pacritinib was administered as front- or second-line treatment and compared to BAT, which was generally ruxolitinib. In the combined pacritinib arms of the 2 PERSIST trials, 18% of the patients achieved SVR ≥35% versus 3% in the BAT arm (P = .001).
Pacritinib may be particularly beneficial in the subgroup of patients with the so-called “myelodepletive phenotype” of MF patients, marked by JAK2 V617F VAF <50%, smaller spleen size, anemia, and thrombocytopenia. The phase III trial PACIFICA trial is presently comparing pacritinib to “physician’s choice” (low-dose ruxolitinib, danazol, steroids, hydroxyurea) in patients with advanced MF and severe thrombocytopenia.
Combination of Ruxolitinib with Agents Targeting Cytopenias
Luspatercept is an activin receptor IIB ligand trap that enhances late-stage erythropoiesis. A pivotal phase III study of luspatercept in combination with ruxolitinib versus ruxolitinib plus placebo in patients with MPN-associated MF who had required 4-12 RBC transfusions in the 12 weeks prior to randomization was recently launched.
The immunomodulatory drug thalidomide is nonmyelosuppressive and may improve anemia and thrombocytopenia in MF, as shown in a phase II study that assessed the combination of ruxolitinib and low-dose thalidomide. Platelet counts increased in 75% of the patients who had thrombocytopenia at baseline.
Combining Agents to Boost Ruxolitinib Responses
New strategies include designing rational combinations with ruxolitinib to boost responses in both the frontline and suboptimal response settings (Table 2). These have included agents that target signal transduction pathways, such as the highly selective phosphatidylinositol 3-kinase delta inhibitor parsaclisib; agents that target the apoptotic pathway, such as navitoclax, the small-molecule, potent inhibitor of the anti-apoptotic B-cell lymphoma-2/extra-large family of proteins; and agents that target epigenetic modifiers, such as the Bromodomain and extra-terminal protein inhibitor pelabresib.
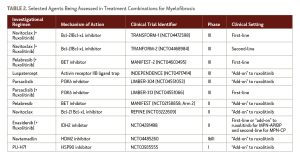
LCL161 is a small-molecule second mitochondria-derived activator of caspases mimetic or antagonist of inhibitor of apoptosis proteins administered orally once weekly. The final results of an investigator-initiated phase II trial that assessed LCL161 in MF patients who had relapsed after/were resistant to, ineligible for or intolerant of JAK inhibitors showed an objective response rate of 30%.
Non–JAK Inhibitor Monotherapy
In the second-line setting, researchers have explored monotherapy with agents other than JAK inhibitors. For example, navtemadlin is a potent, orally bioavailable inhibitor of human double minute 2, a key negative regulator of p53, which is overexpressed in circulating CD34+ cells in MF.
Agents to Enhance Longevity
Imetelstat, a 13-mer oligonucleotide that potently inhibits telomerase activity in MF, demonstrated a survival benefit that compared favorably with matched historical data for BAT in patients with intermediate-2 or high-risk MF that had relapsed or was refractory to JAK inhibitors in the phase II IMbark trial.
Notably, superior OS and clinical outcomes were noted in the higher-risk “triple-negative” MF patients (who are known to have a poor prognosis) compared to the non–triple-negative cohort. At present, the possible survival advantage of imetelstat in the post-JAK inhibitor setting is being compared to BAT (excluding JAK inhibitors), as the primary trial endpoint, in a pivotal international phase III trial in patients with intermediate-2 or high-risk MF who are refractory to JAK inhibitors.
Targeting Bone Marrow Fibrosis
PRM-151 is a recombinant form of the human protein pentraxin-2, a potent antifibrotic agent that prevents and reverses fibrosis at sites of tissue damage. In a phase II trial, PRM-151 in 97 ruxolitinib-intolerant/refractory patients with advanced MF improved the grades of bone marrow fibrosis and collagen at all doses.
In light of the role of TGF-beta in the pathogenesis of bone marrow fibrosis, AVID200 (a highly specific TGF-β1/3 “trap”) is being evaluated in patients with MF who were intolerant/resistant or ineligible to receive ruxolitinib, had bone marrow fibrosis grades 2/3 and platelet counts ≥25 × 109/L. Treatment with AVID200 improved thrombocytopenia and was well tolerated.
Elotuzumab, an anti-SLAMF7 monoclonal antibody, inhibited differentiation of MF patient-derived fibrocytes in vitro and could have the potential to improve or reverse bone marrow fibrosis in MF patients harboring JAK2 V617F.
Other Investigational Agents
Besides the investigational medications reviewed in the previous sections, a multitude of other novel experimental agents have emerged. NS-018 is a promising JAK2 inhibitor that is less immunosuppressive and can be a promising candidate to treat severely thrombocytopenic patients with MF. Tagraxofusp is a CD123-targeted antibody drug conjugate and the first agent that was recently approved for treatment of another hematologic malignancy, blastic plasmatocytoid dendritic cell neoplasm.
The oral selective inhibitor of nuclear export, selinexor, which enhanced growth inhibition and apoptosis of MF CD34+ cells that had been previously exposed to ruxolitinib, is being evaluated in MF patients who relapsed after or were refractory/intolerant to JAK inhibitor therapy.
TL-895 is a highly selective, irreversible Bruton tyrosine kinase inhibitor, which is being assessed as monotherapy in a phase II study in MF patients who have relapsed after or are refractory/intolerant to JAK inhibitor therapy.
Additionally, the safety and efficacy of several novel agents in combination with ruxolitinib will be evaluated in the “add-on” setting in the phase I/II ADORE trial in MF patients who have been on a stable dose of ruxolitinib for 24 weeks or more.
Novel MF agents targeting the programmed cell death 1 (PD-1)/PD ligand-1 (PD-L1) checkpoint axis and therapeutic approaches targeting mutant-CALR induced MPNs have also been explored.
Conclusions and Next Questions
Building on the transformative impact of ruxolitinib on patient care over the last decade, several pivotal phase III trials are underway to assess novel agents in MF, either alone or in rational ruxolitinib-based combination regimens. These investigational regimens are tailored to address urgent unmet clinical needs (anemia, thrombocytopenia, loss of response to JAK inhibitors), boost the activity of ruxolitinib through synergism, or modify the underlying biology of MF and possibly prolong survival.
In the clinical trials that have been conducted for MF to date, the traditional primary and secondary endpoints have been the proportions of patients who achieve SVR ≥35% and a reduction in total symptom score of ≥50%. However, as new promising agents emerge, trials are pivoting to other clinically meaningful endpoints, such as PFS and OS, durability of spleen responses, anemia benefits, bone marrow fibrosis improvement, and reduction of driver mutation allele burden.
After many years with JAK inhibitors being the sole medications to treat MF, it is exciting to witness numerous novel agents in early and advanced clinical development, some raising the bar with regards to therapeutic endpoints and several of them poised to enhance and expand the therapeutic arsenal of MF in the near future. The potential approval of multiple MF medications in the coming months and years will introduce a new challenge for hematologists who will have to optimally sequence these agents and carefully select treatment-naïve patients for upfront combinatorial approaches.






 © 2025 Mashup Media, LLC, a Formedics Property. All Rights Reserved.
© 2025 Mashup Media, LLC, a Formedics Property. All Rights Reserved.