Take-aways:
- Nearly half of patients with relapsed/refractory aggressive B-cell lymphoma who received an anti-CD19 CAR-T therapy relapsed.
- Survival outcomes were poor in patients who had progressive/relapsed disease after receiving CAR-T.
- Relapse within the first 30 days, which occurred in 22% of patients who relapsed, was associated with the worst survival outcomes.
Survival outcomes are typically poor in patients with relapsed/refractory aggressive B-cell lymphoma (BCL) who relapse after treatment with anti-CD19 chimeric antigen receptor (CAR) T cells, according to a multicenter analysis.
While anti-CD19 CAR T cells “represent a major advance in the treatment” of patients with relapsed/refractory aggressive BCL, “a significant number of patients experience failure,” which “remains a major issue, representing an unmet medical need,” according to the study’s first author, Roberta Di Blasi, MD, PhD, of the Hôpital Saint Louis in Paris, France.
For example, multiple clinical trials assessing CAR-T treatments—including the JUILET, ZUMA-1, and TRANSCEND trials—show at least half of patients relapse six months after receiving the therapy. Furthermore, a multicenter French study based on data from the DESCAR-T registry—which collects real-world data on patients who receive commercial CAR-T treatments for up to 15 years after the infusion—indicated more than half of patients experienced treatment failure six months after receiving CAR-T.
Dr. Di Blasi and colleagues conducted the multicenter analysis using the DESCAR-T registry to assess outcomes of patients who progress or relapse after CAR-T, identify prognostic markers, and evaluate potential treatment options for patients who have CAR-T treatment failure.
Study Design and Methods
Researchers studied patients from the DESCAR-T registry, which included those who were eligible to receive CAR-T for a hematologic malignancy covered by the French health care system.
They identified 680 patients with relapsed/refractory aggressive BCL who were registered in DESCAR-T as of August 2018. Most patients (80.1%) received an infusion of a commercially available CAR T-cell product by the time of the analysis in April 2021. The patients received axicabtagene ciloleucel (n=350) or tisagenlecleucel (n=200) and were evaluated at post-infusion days 30, 90, 180, 270, and 360. Patients also received evaluations 18, 24, and 36 months after treatment.
The study’s primary endpoint was overall survival (OS), with secondary endpoints including progression-free survival (PFS), baseline patient characteristics, treatment proposed at failure, response to salvage treatment, and prognostic factors associated with PFS and OS.
The researchers analyzed outcomes according to the time of patient relapse. They defined very-early relapse as occurring between the date of CAR-T infusion and day 30, early relapse as occurring between days 31 and 90, and late relapse as occurring after day 90.
Results
More than half of patients (56%) were considered non-progressive after a median follow-up of 7.9 months. Complete remission occurred in 58% of patients who were non-progressive at that time point, while 11% had partial remission, and 1% had stable disease.
The remaining patients had progressive/relapsed disease after treatment and comprised the population analyzed in the study. The 238 patients with progressive/relapsed disease included 136 who progressed/relapsed at a median follow-up of nine months after receiving axicabtagene ciloleucel and 102 who progressed/relapsed at a median of 7.8 months after receiving tisagenlecleucel.
Nearly 75% of patients who progressed/relapsed presented with diffuse large BCL prior to lymphodepletion, while 66% had progressive disease at the time of infusion. More than half of patients (57.1%) who progressed/relapsed received more than three lines of therapy before CAR-T, while 38.9% of patients had elevated lactate dehydrogenase (LDH) levels.
The median time to treatment failure after infusion was 2.7 months, while the median PFS was 2.8 months (95% CI, 2.4-3.1) from the time of relapse/progression after CAR T-cell infusion. The median OS from the time of relapse/progression after infusion was “consistently poor” at 5.2 months (95% CI, 4.1-6.6), according to Dr. Di Blasi and colleagues.
Nearly half of patients (42.9%) with progressive/relapsed disease had an early relapse, 22.7% had a very-early relapse, and 34.5% had a late relapse (see TABLE 1).
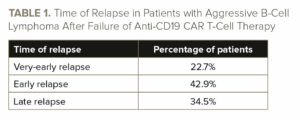
Patients with earlier relapses had reduced PFS and OS compared with patients with later relapses (see TABLE 2).

While 47.9% of patients were alive at six months, only 18.9% of patients who had very-early relapse/progression were alive at that time point.
Several factors were associated with reduced survival. High LDH at infusion (hazard ratio [HR], 3.42; 95% CI, 1.93-6.05; P<.001) and abnormal levels of ferritin at time of infusion (HR, 1.02; 95% CI, 1.00-1.03; P=.01) were both significantly associated with worsened PFS outcomes in a multivariate analysis. Elevated LDH (HR, 2.1; 95% CI, 1.16-3.78; P=.01), elevated C-reactive protein (HR, 1.11; 95% CI, 1.04-1.19; P=.003), and very-early disease progression (HR, 2.93; 95% CI, 1.56-5.5; P=.0009) were all significantly associated with worsened OS outcomes in a multivariate analysis.
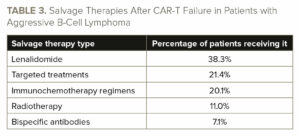
Most patients (64.7%) with progressive/relapsed disease following CAR-T received an additional line of treatment. The most common salvage treatment was lenalidomide (38.3% of patients; see TABLE 3). There was no significant difference in median OS among patients receiving different salvage treatments, nor any significant difference in median PFS.
Limitations and Conclusions
The study’s limitations included a short follow-up period and the potential for missing registry data on patients at the time of relapse.
However, the study’s relatively large sample size allowed researchers to draw conclusions about patterns of treatment outcomes in this population of patients who received commercially available CAR-T treatments in France.
“In conclusion, this DESCAR-T registry study confirms that the outcome of patients at the time of failure after CAR T-cell treatment remains extremely poor, and that this outcome is worse in the event of failure within the first month,” Dr. Di Blasi and colleagues wrote. “Alternative therapeutic strategies (immunotherapy by bispecific antibodies, lenalidomide) may improve PFS rates in these patients. Patients with [relapsed/refractory] aggressive BCL failing after anti-CD19 CAR T-cell treatment constitute an unmet medical need, and further innovative strategies are needed to improve the outcome of such patients.”
Reference
Di Blasi R, Le Gouill S, Bachy E, et al. Outcomes of patients with aggressive B-cell lymphoma after failure of anti-CD19 CAR T-cell therapy: a DESCAR-T analysis. Blood. 2022. doi:10.1182/blood.2022016945






 © 2025 Mashup Media, LLC, a Formedics Property. All Rights Reserved.
© 2025 Mashup Media, LLC, a Formedics Property. All Rights Reserved.