Take-aways:
- Rituximab, lenalidomide, and ibrutinib with sequential addition of chemotherapy led to a 100% ORR and a 94.5% CR rate in patients with newly diagnosed DLBCL.
- The two-year OS rate was 96.6%, while the two-year PFS rate was 91.3%.
- Researchers hope to compare the approach with standard chemotherapy in a phase III trial.
Rituximab, lenalidomide, and ibrutinib with sequential addition of chemotherapy led to a complete response (CR) rate of 94.5% in patients with newly diagnosed diffuse large B-cell lymphoma (DLBCL) in the phase II Smart Start trial.
Jason Westin, MD, MS, of the University of Texas MD Anderson Cancer Center, and colleagues conducted the investigator-initiated, open-label, single-center trial because chemoimmunotherapy approaches for patients with newly diagnosed DLBCL have “remained largely unchanged for decades.”
While approximately 60% of patients receiving a rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone regimen have a curative outcome, 40% of patients develop refractory or relapsed disease despite treatment. Due to this, there is an “unmet need to identify novel approaches to incorporate new drugs in first-line treatment,” Dr. Westin and colleagues wrote.
The researchers developed the Smart Start trial because previous studies showed rituximab, lenalidomide, and ibrutinib may have synergistic activity. In previous studies, the combination led to an overall response rate (ORR) of 65% in patients with non-germinal center B-cell-like DLBCL, which increased to 71% when patients additionally received etoposide, doxorubicin, and vincristine, with prednisone and cyclophosphamide.
The trial is the first to the investigators’ knowledge to “evaluate a targeted therapy combination without chemotherapy in patients with newly diagnosed DLBCL” and will “set the stage for the development of future trials to evaluate additional targeted therapy combinations in DLBCL,” the study’s authors wrote.
Study Design
The trial comprised 60 patients with newly diagnosed and previously untreated non-germinal center B-cell-like DLBCL. The median patient age was 63.5 years, with 28% of patients aged 70 years or older. The median time from diagnosis to treatment was 28 days. Less than half of patients (42%) were high risk according to the revised international prognostic index, while 62% had overexpression of MYC and BCL2 proteins.
All patients received rituximab 375 mg/m2 intravenously once on day one, lenalidomide 25 mg once daily on days one through 10, and ibrutinib 560 mg once daily continuously for each 21-day cycle. After two cycles of rituximab, lenalidomide, and ibrutinib, patients received standard chemotherapy in addition to the triplet treatment for six additional cycles. Treating physicians could select a chemotherapy regimen of rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone or cyclophosphamide, doxorubicin, vincristine, and prednisone.
Results
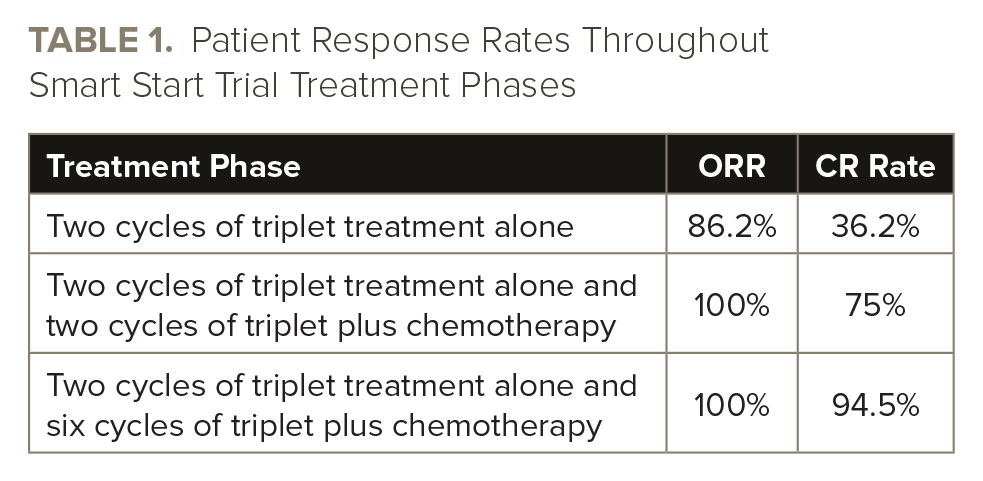
There was no difference in response rates between patients who received chemotherapy with rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone or cyclophosphamide, doxorubicin, vincristine, and prednisone. See TABLE 1 for data on patient response rates.
The two-year progression-free survival (PFS) rate was 91.3% (95% CI, 84.3-98.9), and the median PFS was not reached at a median follow-up of 31 months. The two-year overall survival (OS) rate was 96.6% (95% CI, 92-100), and the median OS was not reached at a median follow-up of 31 months.
Nausea, peripheral sensory neuropathy, diarrhea, and mucositis were the most common adverse events. Febrile neutropenia occurred in 38% of patients, including in 24% of patients receiving cyclophosphamide, doxorubicin, vincristine, and prednisone and in 52% of patients receiving rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone. Atrial fibrillation occurred in 12% of patients, while a grade 3 rash occurred in 16%.
The researchers reported two deaths, both due to infections, as well as two additional deaths, one from disease progression and one from unrelated malignancy, occurring two years after the patients enrolled in the trial.
Limitations and Conclusions
The study’s results were “potentially influenced” by its relatively small sample size—which came from a single tertiary referral center—and “warrant further validation,” the study’s authors wrote, noting that their “approach has been preliminarily replicated by other investigators, and future similar trials are planned.”
“The potential for targeted therapy combinations to reduce or remove the need for chemotherapy is further being explored in an ongoing clinical trial that uses response to targeted therapy to modify the amount of chemotherapy delivered, with the planned next steps if successful of conducting a phase III trial comparing this approach with standard chemotherapy,” Dr. Westin and colleagues wrote. “This design concept could allow for unique targeted therapy combinations to be used in different DLBCL subsets with a single control arm.”
The Smart Start trial’s design and results also can inform other future clinical trials and therapeutic approaches to DLBCL.
The research was supported by Conquer Cancer, the Schweitzer Family Foundation, Celgene, and Janssen.
Reference
Westin J, Davis RE, Feng L, et al. Smart Start: rituximab, lenalidomide, and ibrutinib in patients with newly diagnosed large B-cell lymphoma. J Clin Oncol. 2022. doi:10.1200/JCO.22.00597






 © 2025 Mashup Media, LLC, a Formedics Property. All Rights Reserved.
© 2025 Mashup Media, LLC, a Formedics Property. All Rights Reserved.