The combination of venetoclax and the anti-CD20 monoclonal antibody obinutuzumab with or without ibrutinib outperformed chemotherapy in patients with chronic lymphocytic leukemia (CLL), according to a phase III study.
“Venetoclax [plus] obinutuzumab with or without ibrutinib was superior to chemoimmunotherapy as first-line treatment in fit patients with CLL,” the authors, led by Barbara Eichhorst, MD, of the University Hospital of Cologne in Germany, wrote.1
The large-scale, phase III GAIA/CLL13 trial was conducted across nine European countries and Israel. It enrolled 920 fit patients with CLL who did not have TP53 mutations. Patients were randomly assigned to four groups (see TABLE 1).

Patients received either six cycles of chemoimmunotherapy or 12 cycles of venetoclax in combination with either rituximab, obinutuzumab, or obinutuzumab and ibrutinib. The primary endpoints of the study were undetectable measurable residual disease (MRD; sensitivity, <10−4), as assessed by flow cytometry in peripheral blood at month 15, and progression-free survival (PFS).
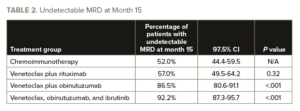
Dr. Eichhorst and colleagues reported that at month 15, the percentage of patients with undetectable MRD was higher in the venetoclax plus obinutuzumab group and in the venetoclax plus obinutuzumab and ibrutinib group than in the chemoimmunotherapy group, but it was not significantly higher in the venetoclax plus rituximab group (see TABLE 2).
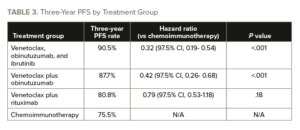
Grade 3 and 4 infections were more common with chemoimmunotherapy (18.5%) and in the venetoclax, obinutuzumab, and ibrutinib arm (21.2%) than in the venetoclax plus rituximab arm (10.5%) or the venetoclax plus obinutuzumab arm (13.2%), Dr. Eichhorst and colleagues reported. See TABLE 3 for the three-year PFS by treatment group.
In all four groups, the treatment was temporary, in contrast to earlier studies that continued either venetoclax or ibrutinib, which prolongs exposure to side effects, dramatically increases the costs, and inevitably results in development of resistance, the authors reported in the press release.
“This study shows that with clever temporary and safe combinations, you can allow patients to be treatment-free in the long-term, with a much lower chance of developing resistance,” Arnon Kater, MD, PhD, a Professor of Hematology at Amsterdam UMC and Chair of the HOVON CLL study group, said in a press release.2
Kater added that it might be possible to stop the combination therapy earlier than after one year.
“We now want to investigate this,” Kater said. “This not only reduces side effects, but also health care costs.”
This study was partially funded by AbbVie.
References
- Eichhorst B, Niemann CU, Kater AP, et al. First-line venetoclax combinations in chronic lymphocytic leukemia. N Engl J Med. 2023;388(19):1739-1754. doi:10.1056/NEJMoa2213093
- Combination therapy outperforms chemotherapy in patients with chronic lymphocytic leukaemia. EurekAlert. May 10, 2023. Accessed May 16, 2023. https://www.eurekalert.org/news-releases/988803






 © 2025 Mashup Media, LLC, a Formedics Property. All Rights Reserved.
© 2025 Mashup Media, LLC, a Formedics Property. All Rights Reserved.