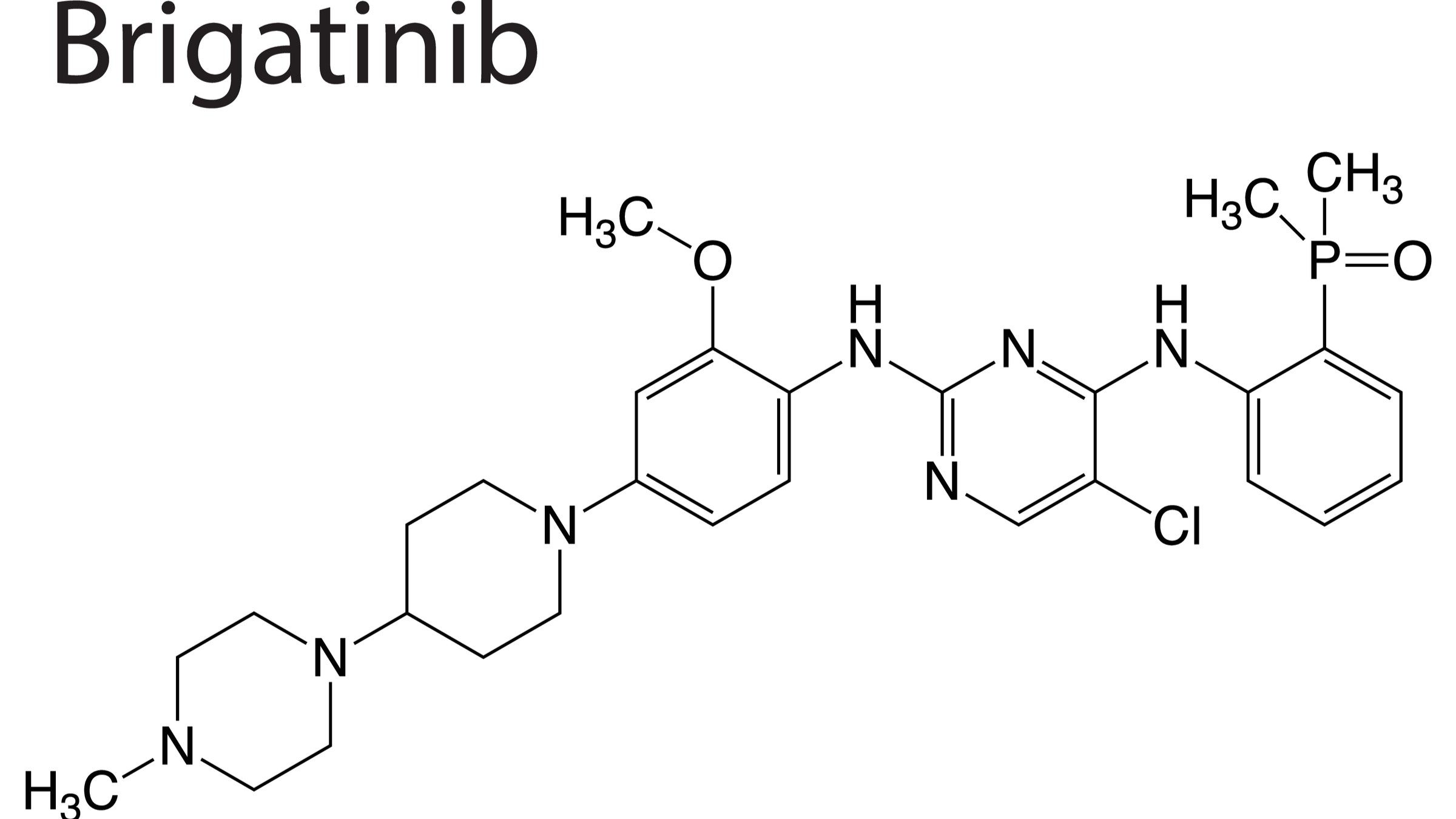
Treatment with brigatinib, an anaplastic lymphoma kinase (ALK) inhibitor, achieved promising responses in patients with ALK-positive anaplastic large-cell lymphoma (ALCL) who failed brentuximab vedotin, according to a case report in the New England Journal of Medicine led by Layla Veleanu, PhD, of the Institut Necker Enfants Malades.
The report noted that ALK inhibitors were initially developed to treat ALK-positive non-small cell lung cancer. In that setting, next-generation ALK inhibitors, including brigatinib, alectinib, and lorlatinib, improved survival versus the first-generation inhibitor crizotinib and were effective in crizotinib-refractory patients.
Additionally, studies on ALK inhibitors in ALK-positive ALCL were mostly with crizotinib, and most patients had not previously received brentuximab vedotin, which is not consistent with current treatment guidelines, according to the report.
Based on these data, Dr. Veleanu and colleagues evaluated off-label brigatinib 180 mg once daily after a seven-day lead-in period of 90 mg once daily in patients with ALK-positive ALCL who previously failed brentuximab vedotin.
The study enrolled 15 patients, of whom four had also previously failed crizotinib; three had crizotinib-resistant disease and one had crizotinib-sensitive disease but relapsed after discontinuing treatment.
After treatment with brigatinib, a best objective response occurred in 93% (n=14) of patients, with a complete response in 73% (n=11). After a median follow-up of 26 months, two-year progression-free survival was 73% and two-year overall survival was 87%. The time to complete response ranged from eight to 325 days.
ALK-associated measurable residual disease in blood was correlated with response. No patients permanently discontinued treatment due to adverse events.
Seven responders went on to receive allogeneic stem cell transplantation. Four patients had disease progression or relapse. Two of the crizotinib-resistant patients and the crizotinib-sensitive patient achieved a complete response to brigatinib, while the remaining crizotinib-resistant patient had a partial response.
“Thus, in comparison with historical controls, the findings of our study support brigatinib as an attractive treatment option in patients with ALK-positive ALCL after the failure of brentuximab vedotin treatment,” Dr. Veleanu concluded.
Reference
Veleanu L, Lamant L, Sibon D. Therapeutic Strategy Project for Adult ALK+ ALCL. Brigatinib in ALK-positive ALCL after failure of brentuximab vedotin. N Engl J Med. 2024;390(22):2129-2130. doi:10.1056/NEJMc2402419












 © 2025 Mashup Media, LLC, a Formedics Property. All Rights Reserved.
© 2025 Mashup Media, LLC, a Formedics Property. All Rights Reserved.