
In a pooled analysis of 10 clinical trials, researchers reported that dose reduction of ibrutinib may be an effective strategy to mitigate the risk of cardiac adverse events (AE) from recurring or worsening in patients with B-cell malignancies.
The study, led by Deborah Marie Stephens, MD, of the Huntsman Cancer Institute at the University of Utah, evaluated 222 patients with grade 1-4 cardiac AEs. Data were pooled for patients treated with ibrutinib from 10 studies, including chronic lymphocytic leukemia (n=781), mantle cell lymphoma (n=250), marginal zone lymphoma (n=63), and Waldenström macroglobulinemia (n=169).
The occurrence of both initial and recurrent cardiac AEs was identified by preferred terms within the cardiac disorders system organ class. Recurrence was defined as an AE of the same or worse grade, and the measurement period extended up to 30 days after the last dose of ibrutinib or the start of the next-line therapy, whichever came earlier.
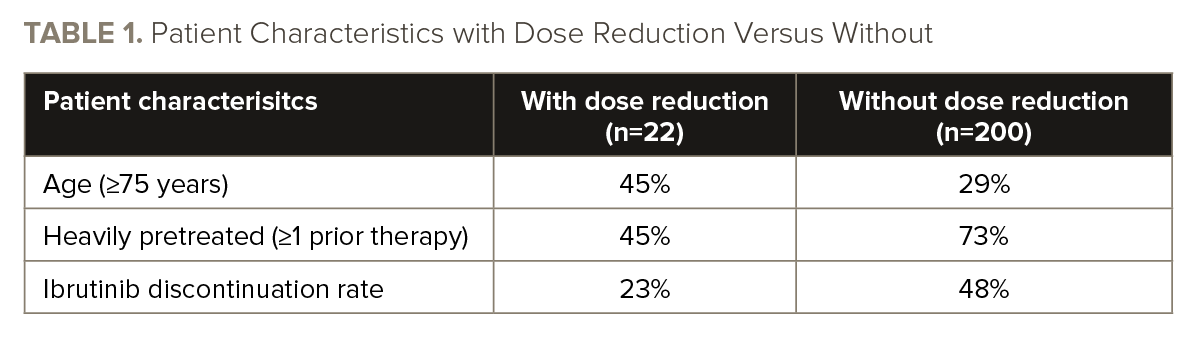
Of the evaluable patients, 22 (10%) had ibrutinib dose reduction to 420 mg (n=3), 280 mg (n=10), or 140 mg (n=9) after a cardiac AE (TABLE 1).
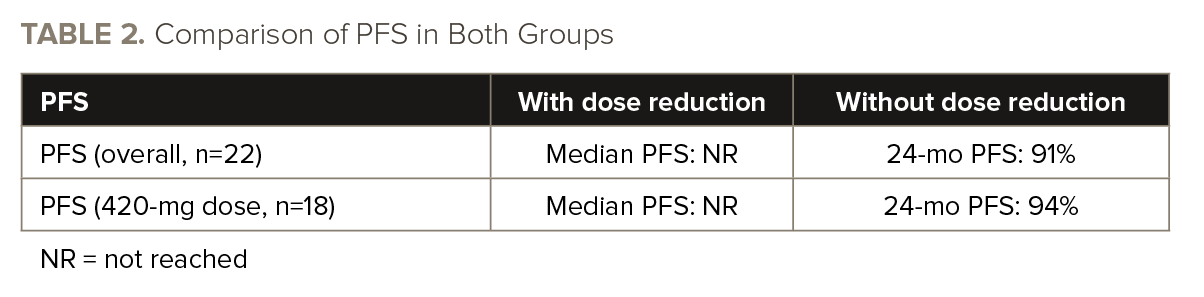
The recurrence of cardiac AEs was observed in 14% of patients with dose reductions, while it occurred in 18% of patients without dose reductions. When specifically considering serious cardiac AEs, the recurrence rate was 5% in patients with dose reductions, compared to 10% in patients without dose reductions. There were zero deaths due to cardiac AE recurrence. TABLE 2 shows PFS in both treatment arms.
“Dose reduction for cardiac AEs may enable [patients] to continue to benefit from long-term ibrutinib and mitigate the risk of cardiac AE recurrence or worsening,” the authors concluded.
Reference
Stephens DM, Brown JR, Ma S, et al. Ibrutinib dose modifications for management of cardiac adverse events in patients with B-cell malignancies: pooled analysis of 10 clinical trials. Abstract #7538. Presented at the 2023 American Society of Clinical Oncology Annual Meeting; June 2-6, 2023; Chicago, Illinois.






 © 2025 Mashup Media, LLC, a Formedics Property. All Rights Reserved.
© 2025 Mashup Media, LLC, a Formedics Property. All Rights Reserved.