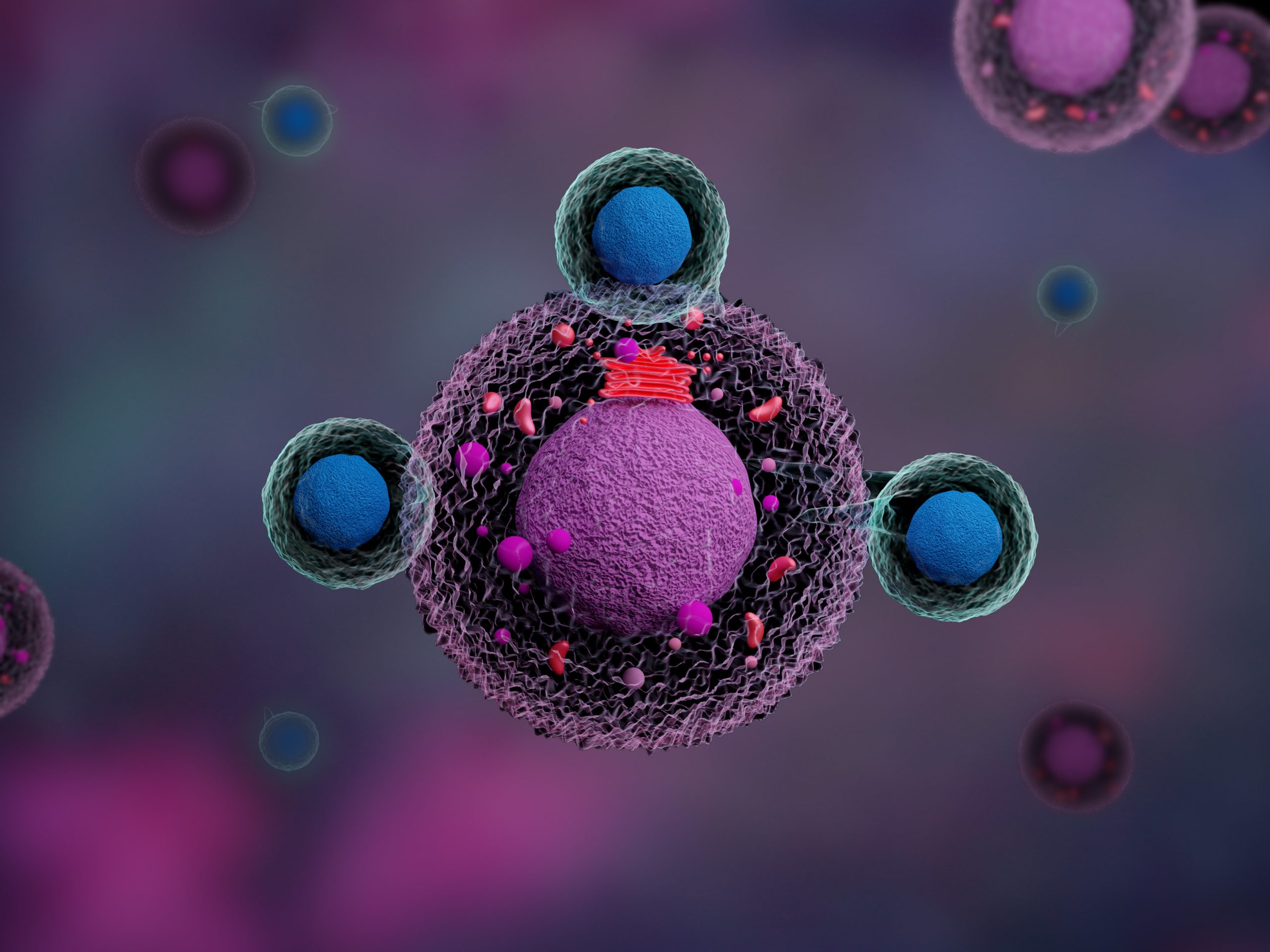
The European Medicines Agency (EMA) has validated a type II variation for the extension of the indication for lisocabtagene maraleucel to treat adult patients with diffuse large B-cell lymphoma (DLBCL), high grade B-cell lymphoma (HGBCL), primary mediastinal large B-cell lymphoma (PMBCL), and follicular lymphoma grade 3b (FL3b) who are refractory or have relapsed within 12 months of initial therapy and are candidates for hematopoietic stem cell transplant.
The application was based on the phase III TRANSFORM study in which lisocabtagene maraleucel, a CD-19-directed chimeric antigen receptor (CAR) T-cell therapy with a 4-1BB costimulatory domain that enhances the expansion and persistence of the CAR T-cells, outperformed the current standard of care with statistically significant improvement in event-free survival.
The therapy is already approved by the U.S. Food and Drug Administration for the treatment of adult patients with relapsed or refractory large B-cell lymphoma after two or more lines of systemic therapy, including DLBCL not otherwise specified (as well as DLBCL arising from indolent lymphoma), HGBCL, PMBCL, and FL3b.
Source: Business Wire press release, June 2022


 © 2025 Mashup Media, LLC, a Formedics Property. All Rights Reserved.
© 2025 Mashup Media, LLC, a Formedics Property. All Rights Reserved.