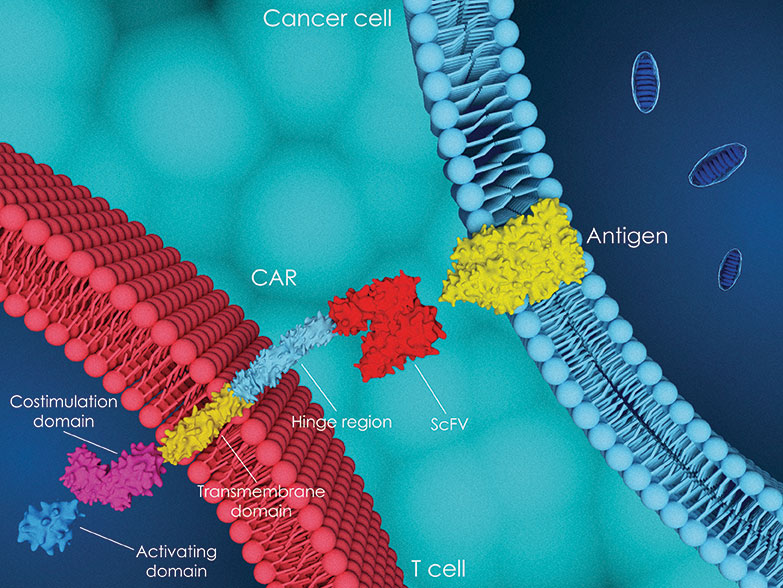
CAR T-cell therapy
Next-line CAR-T or venetoclax-based therapy led to longer OS than other approved next-line treatments.
Autologous chimeric antigen receptor (CAR) T-cell therapies have shown “unprecedented efficacy” in patients with ...
Carlos de Lima, MD, stopped by the Blood Cancers Today booth at the 2022 SOHO Annual Meeting to give his advice...
Advertisement
Blood Cancers Today Editor-in-Chief Sagar Lonial, MD, FACP, provides an update...
As treatments for CLL evolve, new challenges have arisen to address the disease’s resistance to targeted therapies.
In his President's Letter, Moshe Talpaz, MD, discusses all of the treatment progress made for hematologic malignancies.
Advertisement
Blood Cancers Today spoke with clinicians about the two available second-line chimeric antigen receptor T-cell therapies...
CART-ddBCMA demonstrated a 100% overall response rate in patients with relapsed/refractory multiple myeloma.
Doris Hansen, MD, of the Moffitt Cancer Center, spoke about the research during an oral abstract presentation at IMS.
A single infusion of ciltacabtagene autoleucel “resulted in deep and durable responses" in CARTITUDE-2’s cohort A.
The European Medicines Agency’s (EMA) Committee for Medicinal Products for Human Use has issued...
The largest study yet comparing chimeric antigen receptor (CAR) T-cell therapy with the previous standard of care (SOC)...
In second-line therapy for relapsed/refractory LBCL, which patients would you consider eligible for CAR T-cell therapy...
The CAR T-cell therapy received accelerated approval for adults with R/R FL after two or more lines of systemic therapy.
This article discusses managing the most difficult cases of CLL.
Researchers have demonstrated the clinical importance of COVID-19 vaccination after anti-CD19 chimeric antigen receptor (CAR) ...
The FDA has granted Fast Track Designation and Rare Pediatric Disease Designation for WU-CART-007.
Axicabtagene ciloleucel, an autologous anti-CD19 CAR T-cell treatment, improved quality of life compared to standard of care ...
Axicabtagene ciloleucel is the most cost-effective chimeric antigen receptor (CAR) T-cell therapy for patients with relapsed ...
The European Medicines Agency validated a type II variation for the extension of the indication for lisocabtagene maraleucel ...







 © 2025 Mashup Media, LLC, a Formedics Property. All Rights Reserved.
© 2025 Mashup Media, LLC, a Formedics Property. All Rights Reserved.