
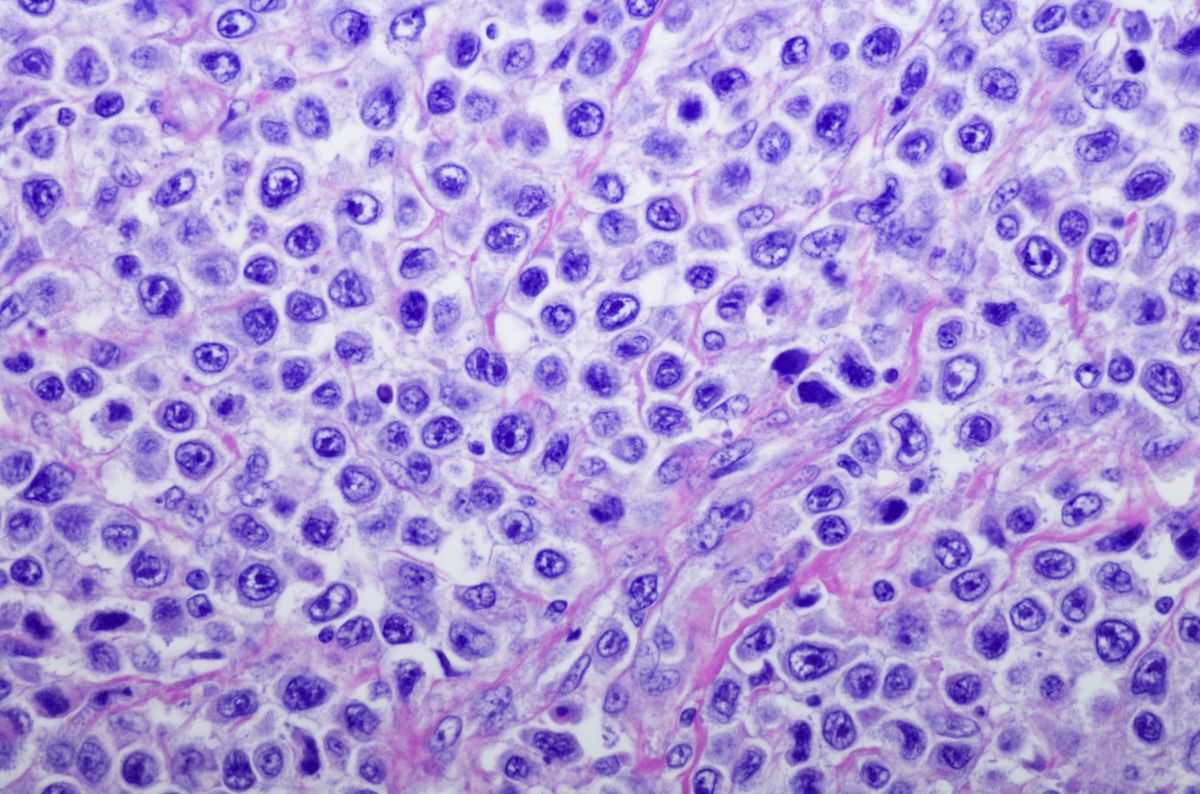
The U.S. Food and Drug Administration (FDA) has cleared an investigational new drug (IND) application for ACE1831, an anti-CD20 armed allogeneic gamma delta T-cell therapy against non-Hodgkin lymphoma (NHL) developed by Acepodia Biotech, Inc.
The approval will initiate a phase I, first-in-human, multicenter clinical study of ACE1831 in patients with NHL.
“Based on ACE1831’s encouraging preclinical data, we believe that our antibodyarmed [gamma delta] T-cell therapy has the potential to provide additional treatment options for patients with NHL,” Sonny Hsiao, PhD, Chief Executive Officer of Acepodia, said in a statement.
Source: Acepodia news release, June 2022





 © 2025 Mashup Media, LLC, a Formedics Property. All Rights Reserved.
© 2025 Mashup Media, LLC, a Formedics Property. All Rights Reserved.