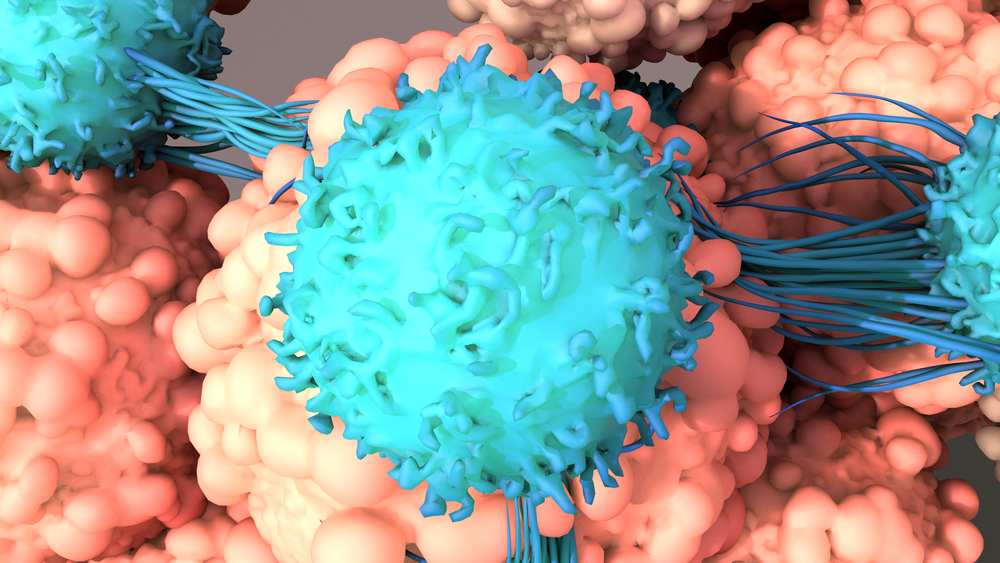
The U.S. Food and Drug Administration (FDA) has granted Fast Track Designation and Rare Pediatric Disease Designation for WU-CART-007 for the treatment of relapsed or refractory (R/R) T-cell acute lymphoblastic leukemia (T-ALL) and acute lymphoblastic lymphoma.
WU-CART-007 is an allogeneic, fratricide-resistant CD7-targeted chimeric antigen receptor (CAR) T-cell therapy engineered to overcome the technological challenges of harnessing CAR T-cells to treat CD7-positive hematological malignancies. It is currently being evaluated for safety and efficacy in patients with R/R T-ALL and ALL in a global, open-label, first-in-human, phase I/II study.
WU-CART-007 is manufactured using healthy donor-derived T cells to eliminate the risk of malignant cell contamination historically observed in the autologous CAR-T setting. Wugen Inc., the developer of WU-CART-007, reported that the company used CRISPR/Cas9 gene editing technology to delete CD7 and the T-cell receptor alpha constant, preventing CAR T-cell fratricide and mitigating the risk of graft-versus-host disease.





 © 2025 Mashup Media, LLC, a Formedics Property. All Rights Reserved.
© 2025 Mashup Media, LLC, a Formedics Property. All Rights Reserved.