
Based on the LLS Professional Education Webcast: Multiple Myeloma: Diagnosis, Treatment, and Side Effects Management
Multiple myeloma (MM) is a malignancy of the plasma cells. A plasma cell is a type of white blood cell that develops from activated B-cells in response to an encounter with an antigen, such as a vaccine, or viruses and bacteria invading the body. These new plasma cells are dedicated to producing large quantities of a single immunoglobulin: an antibody that targets the original antigen. As the abnormal or malignant plasma cells multiply, they accumulate in the bone marrow and cause bone pain, bone loss, and fractures, as well as outnumbering the healthy blood-forming cells, compromising healthy blood cell production. This leads to low blood counts, resulting in anemia (due to reduced red blood cell count) and leukopenia (low white blood cell count), increasing the risk of infections and thrombocytopenia (low platelet count), which may cause easy bruising.
People with MM develop tumors in more than one location inside and sometimes outside the bone marrow, hence the name “multiple” myeloma. In most patients the malignant T-cells also produce excessive amounts of abnormal antibodies called M-protein (monoclonal protein or immunoglobulin, M-spike, paraprotein) that accumulate in the organs, most often affecting kidney function. The paraproteins can also cause direct damage to the nerves in the peripheral nervous system, leading to peripheral neuropathy. These proteins are detectable in the blood or urine and may be intact monoclonal immunoglobulins made of a combination of the immunoglobulin heavy chains and immunoglobulin light chains or comprised of only light chains.
Multiple myeloma is the second most common blood cancer overall and the most common blood cancer in people of African descent. In 2021, the estimated number of new MM cases in the United States will be over 34,000, with more than 12,000 deaths expected to occur. There is a significant difference in incidence and mortality between different patient populations (age, gender, race, etc.). Less than 1% of cases are diagnosed in people aged younger than 35 years. Most people diagnosed with MM or the precursor disease are at least 65 years old. Incidence and mortality are higher for men, and myeloma is about twice as common in people with African heritage (African Americans or Afro-Caribbeans). Family history is also a known risk factor: first-degree relatives of people with MM have a two- to four-fold increased risk of developing the disease.1,2
MM is a heterogeneous and genetically complex disease that develops via a multistep process: plasma cells accumulate genetic alterations over time due to events such as somatic mutations as well as epigenetic and chromosomal copy-number changes, all driving its progression. This process permits myeloma to have distinct and identifiable clinical stages. Almost all MM cases are preceded by the pre-malignant plasma cell disorders monoclonal gammopathy of undetermined significance (MGUS) and/or smoldering multiple myeloma (SMM).3,4 Both are characterized by the presence of M-protein in the blood and are largely asymptomatic, but in case of SMM there is evidence of plasma cell bone marrow infiltration. MGUS is present in around 3% of the general white population aged 50 years and older, with people of African ancestry showing two- to three-fold higher prevalence. Other risk factors include older age, gender, exposure to pesticides, and family history of MGUS or MM. In MGUS, an abnormal line of antibody-producing plasma cells begins to produce monoclonal antibody proteins (M-proteins). People who have MGUS may be at increased risk for MM. The etiology of MGUS is currently unknown, as is why some people with MGUS develop MM and others do not. Risk of progression of MGUS to active malignancy occurs at a rate of 1% per year. In contrast with MM, which causes a wide array of symptoms, MGUS is an asymptomatic plasma cell dyscrasia.5 SMM represents a transitional stage between MGUS and active MM. It is a heterogeneous disease with a 10% risk of progression during the first five years after diagnosis, 3% between five to 10 years after diagnosis, and 1% 10 years after diagnosis.6
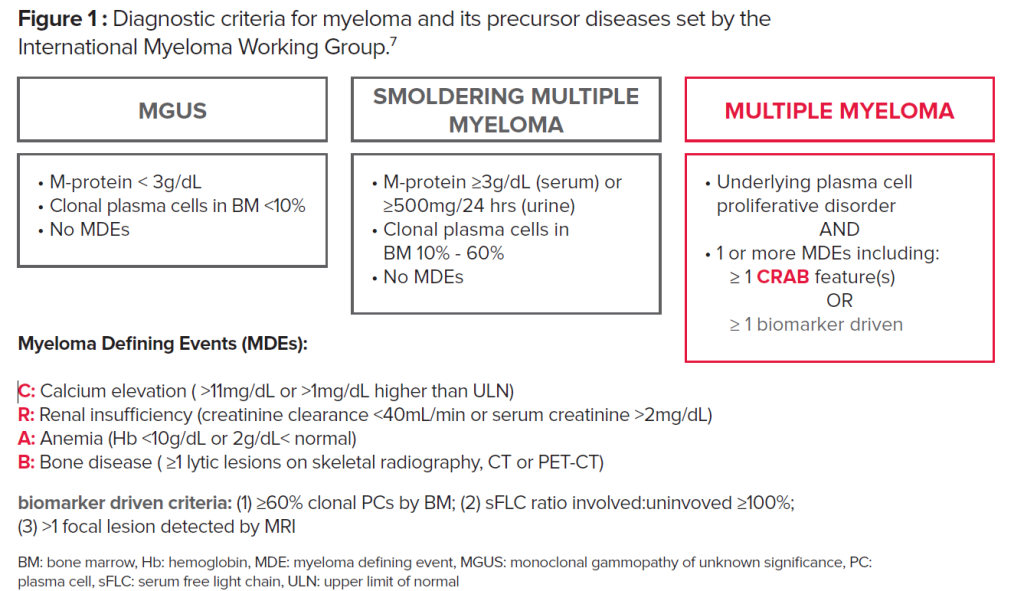
The current diagnostic criteria for myeloma and its precursor diseases follow the guidelines set by the International Myeloma Working Group (IMWG) (Fig. 1). These updated guidelines signal a paradigm shift in the approach to myeloma diagnosis. Historically, the diagnosis of MM required the presence of end-organ damage known as the CRAB criteria (increased Calcium level, Renal dysfunction, Anemia, and destructive Bone lesions). The updated IMWG criteria outlines three myeloma defining events (MDEs): 1) clonal bone marrow plasma cells ≥60%, 2) abnormal serum free light chain (sFLC) ratio ≥100 (involved kappa) or <0.01 (involved lambda), and 3) one or more focal >5 mm lesions on MRI scans. The presence of at least any one of these markers is considered sufficient for a diagnosis of MM, independent of symptoms or CRAB features.7
We will continue the Myeloma 101 series in the next issue of Multiple Myeloma Today.
Orsi Giricz, PhD, joined the LLS Research Team as the Director of Research Programs in September 2018. Dr. Giricz brings more than 15 years of academic research experience to the organization, in both solid and liquid tumors. She works with other LLS scientists to develop and implement new transformative research programs while also monitoring/evaluating LLS’ currently supported grants. She is the program lead for the Blood Cancer Discoveries Grants, as well as all the myeloma SCOR (Specialized Center of Research) grants.
Prior to joining LLS, Dr. Giricz was a Faculty Associate and Senior Scientist at the Albert Einstein College of Medicine, where her research focused on different blood cancers. She has published in numerous peer-reviewed journals and still actively contributes to on-going blood cancer research.
Saad Zafar Usmani, MD, received his medical education at Allama Iqbal Medical College in Lahore, Pakistan. He completed a residency in internal medicine at Sinai-Grace Hospital/Wayne State University in Detroit, Michigan, and a fellowship in hematology and oncology at the University of Connecticut Health Center in Farmington, CT. Dr. Usmani joined the faculty of the Levine Cancer Institute in July 2013; he also currently holds an academic appointment as Clinical Professor of Medicine at the University of North Carolina-Chapel Hill School of Medicine. Previously, Dr. Usmani served as an Assistant Professor of Medicine at the University of Arkansas for Medical Sciences in Little Rock, Arkansas, and Director of Developmental Therapeutics at the Myeloma Institute for Research & Therapy.
References
- Smith CJ, Ambs S, Landgren O, Biological determinants of health disparities in multiple myeloma. Blood Cancer Journal, 2018.8:85.
- Siegel, R.L., Miller KD, Fuchs HE, et al., Cancer Statistics, 2021. CA: A Cancer Journal for Clinicians, 2021;71:7-33.
- Landgren O, Kyle RA, Pfeiffer RM, et al., Monoclonal gammopathy of undetermined significance (MGUS) consistently precedes multiple myeloma: a prospective study. Blood, 2009;113:5412-7.
- Weiss BM,, Abadie J, Verma P, et al., A monoclonal gammopathy precedes multiple myeloma in most patients. Blood, 2009.113:5418-22.
- Kyle, RA, Therneau TM, Rajkumar SV, et al., Prevalence of monoclonal gammopathy of undetermined significance. New England Journal of Medicine, 2006;354:1362-9.
- Rajkumar SV, Landgren O, Mateos MV. Smoldering multiple myeloma. Blood, 2015;125:3069-75.
- Rajkumar SV., Multiple myeloma: 2020 update on diagnosis, risk-stratification and management. American Journal of Hematology, 2020;95:548-67.






 © 2025 Mashup Media, LLC, a Formedics Property. All Rights Reserved.
© 2025 Mashup Media, LLC, a Formedics Property. All Rights Reserved.