Take-aways:
- Patients with bulky stage I/II classic Hodgkin lymphoma who received ABVD underwent PET to determine if they should receive continued ABVD or escBEACOPP plus radiotherapy.
- Most patients were able to avoid escBEACOPP plus radiotherapy based on PET results.
- The estimated three-year PFS rate for all patients in the study was 92.3%.
Positron emission tomography (PET)-adapted therapy was associated with “excellent” progression-free survival (PFS) in patients with bulky stage I/II classic Hodgkin lymphoma, according to a recent study.
Ann LaCasce, MD, of the Dana Farber Cancer Institute, and colleagues conducted the research because patients with bulky stage I/II classic Hodgkin lymphoma are “typically treated with chemotherapy followed by radiation,” but “late effects associated with radiotherapy include increased risk of second cancer and cardiovascular disease.”
They hypothesized a PET-adapted strategy would be “effective and limit the use of mediastinal radiotherapy in this important and particularly high-risk group of patients in terms of late effects.”
Dr. LaCasce and colleagues evaluated the PET-adapted approach in patients with bulky early-stage classic Hodgkin lymphoma, omitting radiotherapy in patients who had interim PET-negative disease and intensifying treatment in those with PET-positive disease.
Phase II Trial Design and Patient Characteristics
The single-arm, phase II trial included patients with bulky disease, defined as a mass >10 cm or one-third of the maximum intrathoracic diameter on a chest x-ray. All patients received two cycles of full-dose doxorubicin, bleomycin, vinblastine, and dacarbazine (ABVD) followed by interim fluorodeoxyglucose PET (PET2). The patients underwent centrally reviewed PET on days 23 to 25 after the first day of cycle two.
Those who had a PET2-negative status, which the study’s authors defined as a score of one to three on the Deauville five-point scale, received four additional cycles of ABVD.
Patients who had a PET2-positive status received four cycles of escalated bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisone (escBEACOPP) followed by 30.6 gray involved-field radiation.
Patients received PET at the end of therapy and received computed tomography (CT) scans every three months for one year, every six months during years two and three, and then annually for a maximum of five years. The PET scans underwent central review by a member of an “expert team of PET-CT readers” with an “adjudicator from the same pool in the case of disagreement with the local physician,” Dr. LaCasce and colleagues wrote.
The study included 94 evaluable patients with a median age of 30 years (range, 18 to 58 years). A little more than half (53%) of patients were female. Nearly all (90%) patients had stage II disease, including 48 (51%) with stage IIB/IIBE disease.
The study’s primary endpoint was the three-year PFS rate. The study’s objectives were to maintain PFS in patients with a PET2-negative status without radiotherapy and improve PFS for patients with PET2-positive status by intensifying chemotherapy with consolidative radiotherapy.
Patient Outcomes with PET-Adapted Approach
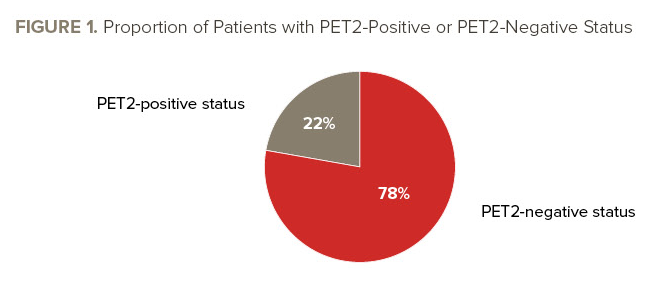
See FIGURE 1 for PET2 results. Patients with a PET2-negative status had a three-year PFS rate of 93.1%, and those with a PET2-positive status had a three-year PFS rate of 89.7%. The estimated three-year PFS rate for all patients was 92.3% (95% CI, 87.0-98.0).
At a median follow-up of 60 months, seven PFS events, including five progressions and two deaths, occurred in the 73 patients with a PET2-negative status. There were two PFS events—both of which were progressions—in the 21 patients with a PET2-positive status.
When the researchers compared the PFS of patients with a PET2-positive status with those who had a PET2-negative status, the estimated hazard ratio was 1.03, which was significantly less than the null hypothesis of 4.1 (P=.04).
The three-year overall survival (OS) rate was 98.6% in those with a PET2-negative status and 94.4% in those with a PET2-positive status. The estimated three-year OS rate for all patients was 97.7% (95% CI, 94.7-100). See TABLE 1 for information on complete response (CR) rates.

At a median follow-up of 69 months for survival, four patient deaths occurred, including one in a patient with a PET2-positive status.
Nearly all (93%) patients who were evaluable for safety completed therapy per the study protocol, including 96% of those with a PET2-negative status and 91% of those with a PET2-positive status.
Neutropenia was the “predominant toxicity” reported by Dr. LaCasce and colleagues, as 9% of patients developed febrile neutropenia.
The researchers did not report any “unexpected” adverse events (AEs) in patients who received ABVD or escBEACOPP. No grade 5 AEs occurred.
Study Meets ‘Primary Goal’
Overall, patient outcomes were “excellent,” the researchers reported, but the study had several limitations, including its moderate size for a phase II study that did not randomize patients to receive standard therapy versus a PET-adapted approach. Furthermore, the sample of patients with a PET2-positive status after two cycles of ABVD was small, the researchers reported.
Despite the limitations, the study “met its primary goal” and has “important implications for clinical practice,” they wrote.
Future studies should evaluate novel agents in patients with early-stage disease, according to LaCasce and colleagues.
“The majority of patients with PET2-negative [status] remained disease-free without the need for high-dose chemotherapy with autologous stem cell transplant,” they concluded. “Future studies should focus on incorporating novel agents in early-stage patients who are unlikely to achieve durable remissions with chemotherapy alone and reduce exposure to regimens such as escBEACOPP, which may have a negative impact on fertility and bone marrow stem cells.”
Reference
LaCasce AS, Dockter T, Ruppert AS, et al. Positron emission tomography-adapted therapy in bulky stage I/II classic Hodgkin lymphoma: CALGB 50801 (Alliance). J Clin Oncol. 2023;41(5):1023-1034. doi:10.1200/JCO.22.00947






 © 2025 Mashup Media, LLC, a Formedics Property. All Rights Reserved.
© 2025 Mashup Media, LLC, a Formedics Property. All Rights Reserved.