
Pola-R-CHP and R-CHOP “demonstrated similar safety profiles” in older adults with previously untreated diffuse large B-cell lymphoma (DLBCL), but the “risk-benefit profile favored” pola-R-CHP, according to results from the phase III POLARIX study.
Bei Hu, MD, of the Levine Cancer Institute and colleagues presented the data during the 2023 American Society of Clinical Oncology Annual Meeting.
The phase III POLARIX study showed a significant improvement in progression-free survival (PFS) with pola-R-CHP over R-CHOP in patients aged 18 to 80 years who had previously untreated DLBCL. The two regimens showed “similar” safety profiles, according to the study’s authors.
However, combination regimens “may be associated with higher rates of toxicity” in patients with previously untreated DLBCL, Dr. Hu and colleagues wrote. Due to this, they compared the efficacy and safety of pola-R-CHP and R-CHOP in patients who were at least 70 years old when they enrolled in the POLARIX trial.
Patients with previously untreated DLBCL were randomized 1:1 to receive six cycles pola-R-CHP or R-CHOP, plus two cycles of rituximab. They analyzed 284 patients for efficacy (pola-R-CHP, n=141; R-CHOP, n=143) and analyzed 280 patients for safety (pola-R-CHP, n=137; R-CHOP, n=143). The median patient age was 74 years (range, 70-80 years), and most patients (69.7%) had an International Prognostic Index score of three to five.
In the group receiving pola-R-CHP, 88.3% of patients received all six doses of polatuzumab vedotin. In the group receiving R-CHOP, 91.6% of patients received all six doses of vincristine.
With a median follow-up of 24.2 months by the data cutoff, the risk of progression, relapse, or death was lower with pola-R-CHP than R-CHOP (hazard ratio [HR], 0.64; 95% CI, 0.41-0.99). See TABLE 1 for data on progression-free survival (PFS), overall survival (OS), and disease-free survival (DFS).
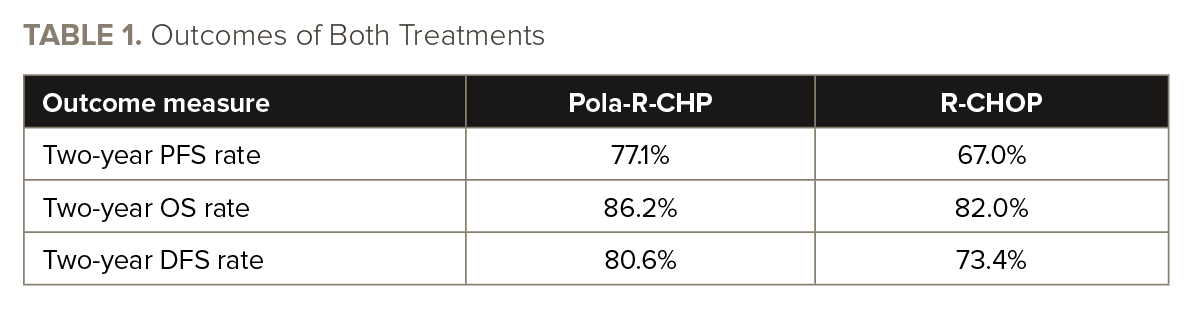
Death by any cause occurred in 14.2% of patients who received pola-R-CHP and in 19.6% of those who received R-CHOP. The “safety profiles were generally comparable” for pola-R-CHP and R-CHOP, according to the study’s authors.
See TABLE 2 for data on adverse events (AEs).
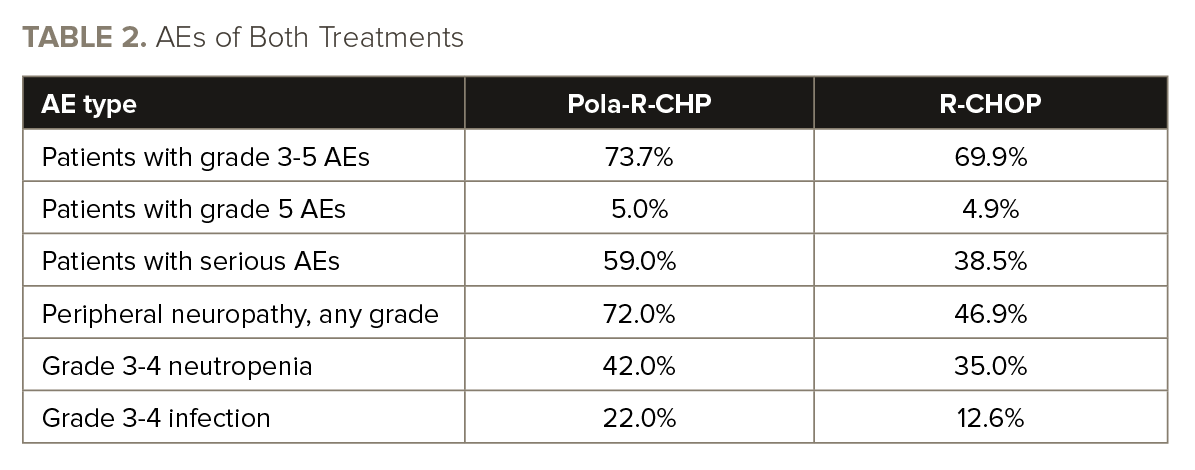
“Pola-R-CHP and R-CHOP demonstrated similar safety profiles in [patients] aged ≥70 years with previously untreated DLBCL,” Dr. Hu and colleagues concluded. “The risk-benefit profile favored pola-R-CHP vs R-CHOP.”
Reference
Hu B, Reagan PM, Sehn LH, et al. Subgroup analysis of elderly patients (pts) with diffuse large B-cell lymphoma (DLBCL) in the phase 3 POLARIX study. Abstract #7518. Presented at the 2023 American Society of Clinical Oncology Annual Meeting; June 2-6, 2023; Chicago, Illinois.






 © 2025 Mashup Media, LLC, a Formedics Property. All Rights Reserved.
© 2025 Mashup Media, LLC, a Formedics Property. All Rights Reserved.