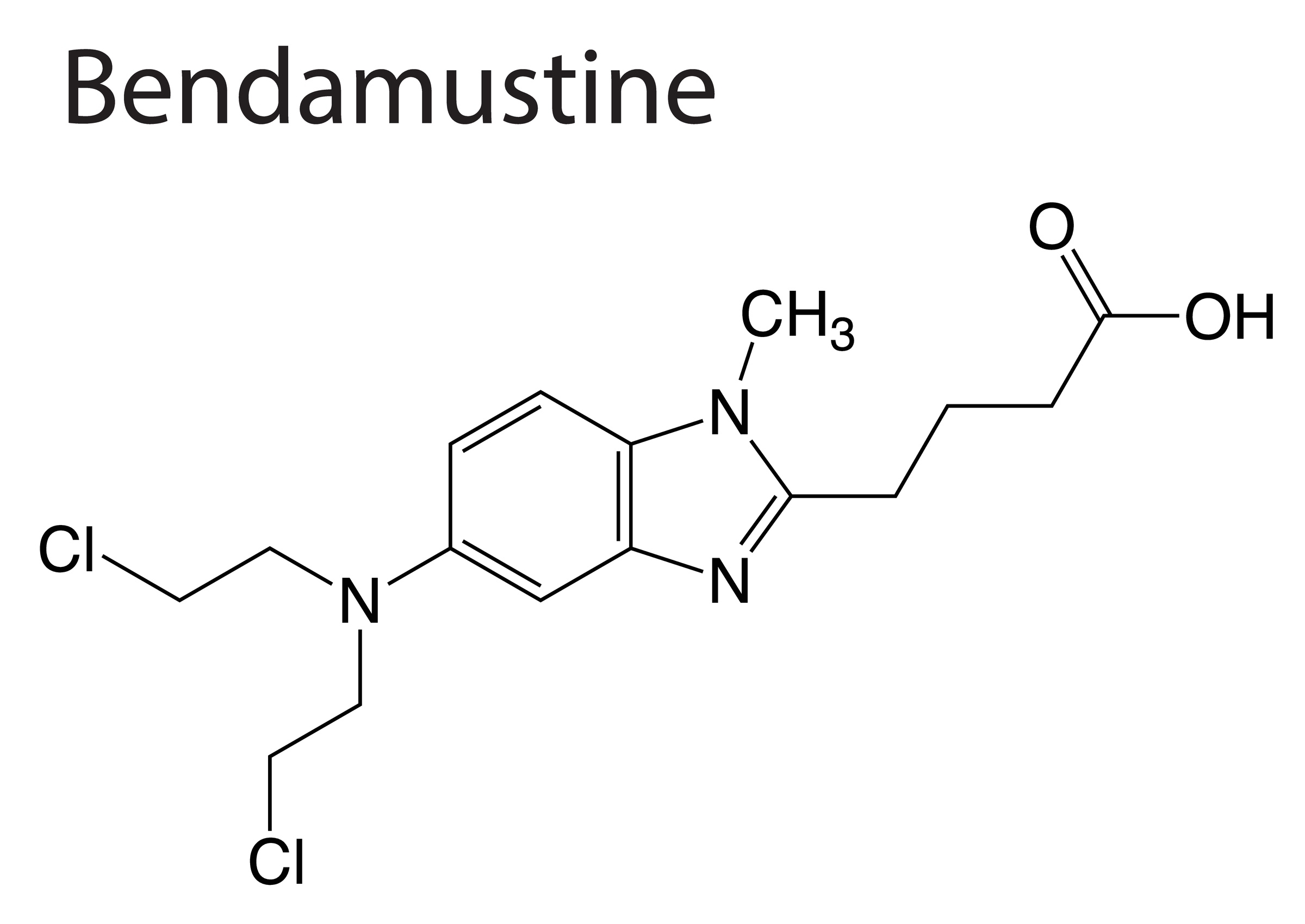
Rates of bendamustine discontinuation and bendamustine-related deaths in real-world patients in the United Kingdom with indolent B-cell non-Hodgkin lymphoma were comparable to those in published data, according to an analysis in Blood Advances.
Additionally, infections and opportunistic infections were common though they often occurred long after treatment was completed, wrote lead author of the study, Rohan Shotton, doctoral fellow at the Christie National Health Service (NHS) Foundation Trust in Manchester, United Kingdom, and colleagues.
The study evaluated 323 patients from nine NHS centers who received at least one dose of bendamustine with or without rituximab. Overall, 96% of patients received bendamustine with rituximab and 46% received rituximab maintenance.
A total of 21.7% of patients experienced serious adverse events (AEs) including infections in 12%. Absolute risk for infections was highest during induction (63%), followed by maintenance (20%), and follow-up (17%), and relative risk was highest during maintenance (54%) followed by induction (34%) and follow-up (28%).
Toxicities related to bendamustine led to permanent discontinuation in 13% of patients and death in 2.8% due to bendamustine-related infections, myelodysplastic syndromes, or cardiac disease. The authors noted use of antimicrobial prophylaxis varied among the patients, and three out of 10 cases of opportunistic infections occurred despite prophylaxis.
Predictors of increased risk for serious AEs included poor preinduction performance status, poor premaintenance, abnormal preinduction total globulins, use of growth factors, and rituximab maintenance.
“In this real-world analysis, bendamustine-related deaths and treatment discontinuation were similar to those of trial populations of younger, fitter patients,” Shotton and colleagues concluded.
Reference
Shotton R, Broadbent R, Alchawaf A, et al. Safety of bendamustine for the treatment of indolent non-Hodgkin lymphoma: a UK real-world experience. Blood Adv. 2024;8(4):878-888. doi:10.1182/bloodadvances.2023011305b






 © 2025 Mashup Media, LLC, a Formedics Property. All Rights Reserved.
© 2025 Mashup Media, LLC, a Formedics Property. All Rights Reserved.