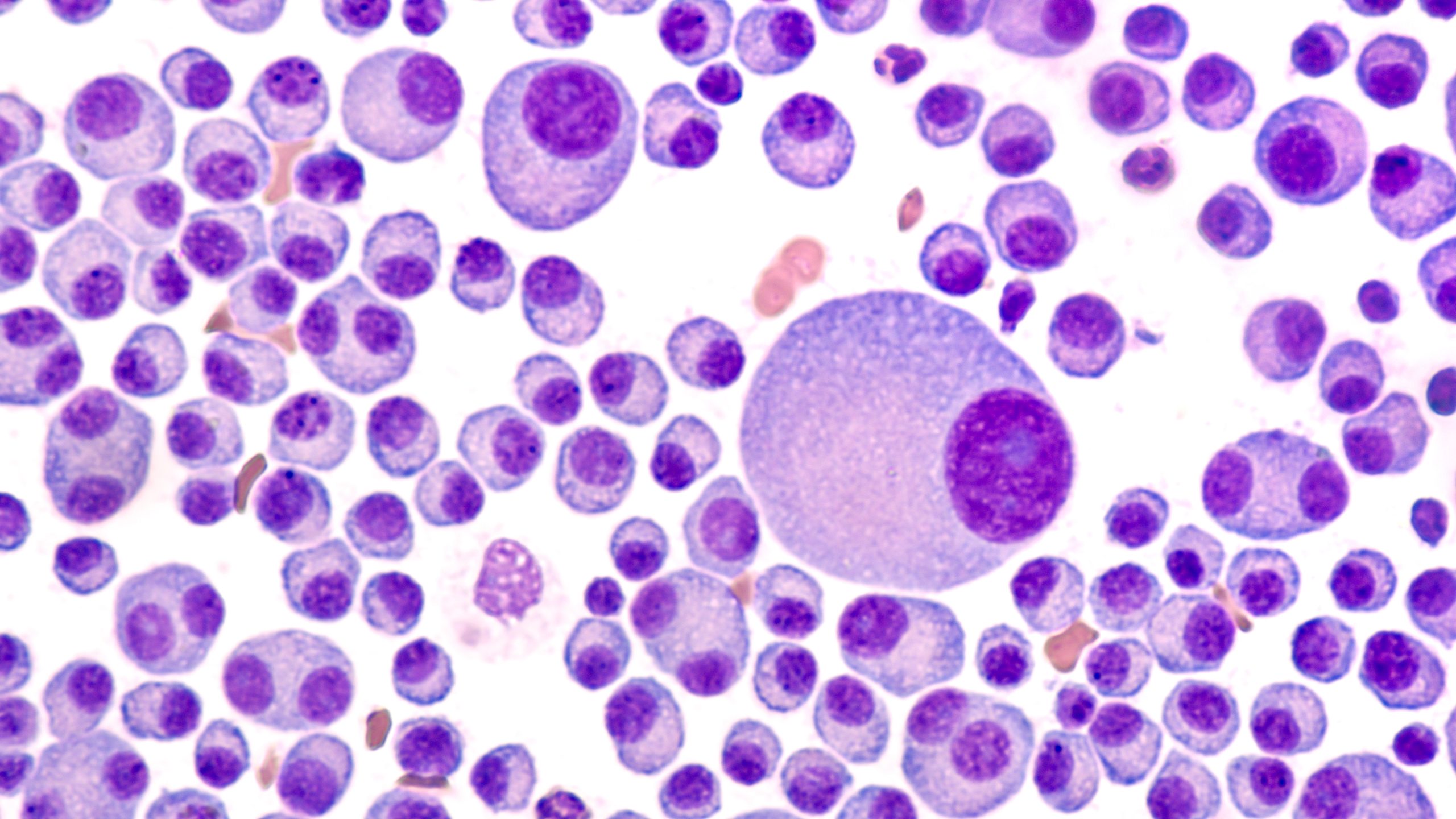
The European Medicines Agency’s (EMA) Committee for Medicinal Products for Human Use (CHMP) has recommended conditional marketing authorization (CMA) for teclistamab as monotherapy for adult patients with relapsed/refractory (R/R) multiple myeloma (MM) who have received at least three prior therapies, including an immunomodulatory agent, a proteasome inhibitor, and an anti-CD38 antibody, and have demonstrated disease progression since last therapy.
The EMA’s CHMP recommendation is based on positive results from the multicohort, open-label, phase I/II MajesTEC-1 study evaluating the safety and efficacy of teclistamab in adults with R/R MM.
Teclistamab targets both B-cell maturation antigen (BCMA) and CD3. It works by redirecting CD3-positive T-cells to BCMA-positive myeloma cells, resulting in T-cell activation and subsequent lysis and death of BCMA-positive cells. This effect occurs regardless of T-cell receptor specificity or major histocompatibility complex class I molecules on the surface of the myeloma cells.
Source: BusinessWire via the Janssen Pharmaceutical Companies of Johnson & Johnson, July 2022



 © 2025 Mashup Media, LLC, a Formedics Property. All Rights Reserved.
© 2025 Mashup Media, LLC, a Formedics Property. All Rights Reserved.