
Take-aways:
- Exposure to thalidomide analogs, particularly lenalidomide, is significantly associated with TP53 mutations in patients with therapy-related myeloid neoplasms.
- Treatment with lenalidomide, but not pomalidomide, provides a selective advantage to preleukemic Trp53-mutated HSPCs.
- These data suggest a “biological rationale” for pomalidomide use in patients who are at a high risk for therapy-related myeloid neoplasms.
A new study showed a significant association between previous lenalidomide treatment and TP53 mutations in patients with therapy-related myeloid neoplasms.
Adam S. Sperling, MD, PhD, of the Dana-Farber Cancer Institute and Brigham and Women’s Hospital, and colleagues conducted the study and published the results in Blood.
Dr. Sperling and colleagues conducted the research because therapy-related myeloid neoplasms, which “arise from selective pressure introduced” by chemotherapy and radiation therapy “represent one of the most devastating consequences of cancer therapy,” as they are frequently resistant to treatment, with a median OS of seven to 14 months.
It is crucial to understand “how individual therapies promote the outgrowth of specific mutant clones” and develop strategies to reduce the risk of therapy-related myeloid neoplasms by modifying therapies, according to the study’s authors.
Consequently, Dr. Sperling and colleagues conducted a systematic analysis of the association between therapy-related myeloid neoplasm genotypes and prior chemotherapy and radiation therapy.
Methodology
The researchers retrospectively reviewed data from 416 patients with therapy-related myeloid neoplasms who were diagnosed per the 2016 World Health Organization classification and treated at a single institution between November 2008 and February 2019.
Of the 416 patients, 40% had therapy-related AML, while 60% had therapy-related myelodysplastic syndromes (MDS). Most patients (63%) had a primary diagnosis of solid tumors, while 37% had non-myeloid hematologic cancers.
Nearly half (45%) of patients received prior treatment with chemotherapy alone, while 17% received radiotherapy alone, and 39% received chemotherapy and radiotherapy. Most patients (83%) did not undergo autologous hematopoietic stem cell transplantation.
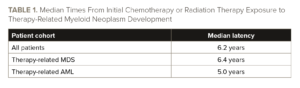
The median latency from initial chemotherapy or radiation therapy exposure to diagnosis was 6.2 years. The latency period was significantly shorter in patients who developed therapy-related AML (median latency, five years) than in those who developed therapy-related MDS (median latency, 6.4 years; P=.0283). See TABLE 1.
The study’s authors used next-generation sequencing to perform mutation analyses on cryopreserved diagnostic bone marrow or peripheral blood samples. They also analyzed a comparison group of 1,021 patients with de novo myeloid neoplasms, including 611 patients with non-treatment-related AML and 410 with de novo MDS who were diagnosed and treated during the same time at the same institution.
Dr. Sperling and colleagues detected somatic mutations in bone marrow or peripheral blood samples from 156 of the 416 patients with therapy-related myeloid neoplasms by using hybrid capture sequencing of coding regions in 300 genes. They used the same technique in the remaining 260 patients to detect somatic mutations in 81 genes.
The researchers also conducted a multiplexed in vivo CRISPR knockout screen in hematopoietic stem and progenitor cells (HSPCs) from mice, generated Hoxb8 cell lines and Hoxb8 cell line experiments, and performed competitive bone marrow transplantation and thalidomide analog treatment in mouse models.
Frequency of Mutations
Nearly half of patients (41%) had a complex karyotype, which was −7/del(7q) in 34%, −5/del(5q) in 32%, and 11q23 rearrangements in 7%. Almost all (85%) patients with therapy-related myeloid neoplasms had at least one detectable mutation, which were primarily in TP53 (37%) and PPM1D (19%). See TABLE 2.

Multi-hit alterations occurred in 61% of TP53-mutated cases in combination with del(17p) or multiple TP53 mutations. Mutations in TET2 occurred in 16% of patients, DNMT3A in 15%, RUNX1 in 13%, ASXL1 in 13%, and SRSF2 in 10%.
The researchers compared mutational frequency between patients with therapy-related AML or MDS and those with non-therapy-related AML or MDS. Mutations in TP53 and PPM1D were significantly more frequent in patients with therapy-related AML or MDS, while mutations in STAG2 and ASXL1 were more common in patients with AML or MDS who did not previously undergo chemotherapy or radiotherapy.
Furthermore, NPM1, IDH1/2, FLT3, CEBPA, and NRAS mutations were enriched in patients who had AML without exposure to chemotherapy or radiotherapy. Mutations in TET2, PHF6, SRSF2, SF3B1, and U2AF1 were more common in patients who had MDS and no prior exposure to chemotherapy or radiotherapy.
Dr. Sperling and colleagues assessed the association between mutations and prior exposure to chemotherapy and/or radiotherapy, finding a significant correlation between complex karyotype and treatment with platinum agents (odds ratio [OR], 1.88; 95% CI, 1.23-2.89). They also found a significant correlation between chromosome 7 abnormalities and alkylating agent treatment (OR, 1.64; 95% CI, 1.08-2.49) or platinum drugs (OR, 1.65; 95% CI, 1.06-2.57).
The researchers also found significant associations between TP53 mutations and proteasome inhibitors (OR, 3.06; 95% CI, 1.52-6.15) and between TP53 mutations and thalidomide analogs (OR, 2.62; 95% CI, 1.36-5.05).
A multivariate logistic regression analysis confirmed the significant association between TP53 mutations and prior exposure to thalidomide analogs (OR, 3.14; 95% CI, 1.60-6.18; P=.0009). It also confirmed the significant association between TP53 mutations and exposure to vinca alkaloids (OR, 1.76; 95% CI, 1.05-2.93; P=.031) and a significant negative association between TP53 mutations and exposure to topoisomerase inhibitors (OR, 0.49; 95% CI, 0.26-0.91; P=.023).
The researchers investigated the effect of lenalidomide on mutant HSPCs because 92% of the thalidomide analog exposure in the patients studied involved lenalidomide. Lenalidomide provided a “selective advantage” to murine Trp53-mutant HSPCs, as lenalidomide, but not pomalidomide, led to outgrowth of Trp53-mutant cells in all blood cell lineages, according to Dr. Sperling and colleagues.
“The primary difference between the activities of lenalidomide and pomalidomide is the degradation of CK1α,” they wrote.
Treatment Implications
The significant association between TP53-mutated therapy-related AML or MDS and prior exposure to thalidomide analogs, particularly lenalidomide, is likely to have treatment and prognostic implications.
For example, the study showed pomalidomide may limit “the selective advantage of TP53-mutant clones,” which provides a “biological rationale” to use it in patients who have a high risk of developing therapy-related myeloid neoplasms, the study’s authors wrote.
“These findings highlight the role of lenalidomide treatment in promoting TP53-mutated [therapy-related myeloid neoplasms] and offer a potential alternative strategy to mitigate the risk of [therapy-related myeloid neoplasm] development,” Dr. Sperling and colleagues concluded.
Reference
Sperling AS, Guerra VA, Kennedy JA, et al. Lenalidomide promotes the development
of TP53-mutated therapy-related myeloid neoplasms. Blood. 2022;140(16):1753-1763.
doi:10.1182/blood.2021014956






 © 2025 Mashup Media, LLC, a Formedics Property. All Rights Reserved.
© 2025 Mashup Media, LLC, a Formedics Property. All Rights Reserved.