Take-aways:
- The triplet regimen of pomalidomide, dexamethasone, and daratumumab demonstrated efficacy immediately following first- or second-line lenalidomide treatment failure.
- With a median follow-up of 28.4 months, ORR was 77.7%, with 52.7% of patients achieving VGPR or better.
- The results of the study support the use of pomalidomide as a foundation for combination therapy in relapsed/refractory MM.
Treatment with the triplet regimen of pomalidomide, dexamethasone, and daratumumab given immediately after early-line lenalidomide-based treatment is safe and effective in patients with relapsed/refractory multiple myeloma (MM) after early-line lenalidomide treatment failure, according to results from the phase II MM-014 trial.
The results, published in Leukemia & Lymphoma by Nizar J. Bahlis, MD, Associate Professor of Hematology and Oncology at the University of Calgary, and colleagues, support the use of pomalidomide as a foundation for combination therapy in relapsed/refractory MM and suggest that it is not necessary to switch from the immunomodulatory agent class following lenalidomide treatment failure.
Treatment with the immunomodulatory agent lenalidomide until disease progression is a standard of care for the treatment of newly diagnosed and relapsed/refractory MM, but for many patients, the disease becomes refractory to lenalidomide or relapses during early treatment lines.
In the United States, the triplet regimen of pomalidomide, dexamethasone, and daratumumab is approved for the treatment of patients with MM who received two or more prior therapies, including lenalidomide and a proteasome inhibitor. Until now, this regimen had not been extensively studied in earlier lines of therapy or in patients who developed lenalidomide-refractory disease immediately prior to administration of the triplet regimen.
Phase II MM-014 Trial Design
The non-randomized, multicenter, multinational, open-label MM-014 study was designed to investigate outcomes of sequencing pomalidomide-based therapy immediately following lenalidomide treatment failure in early treatment lines in 49 study sites across the United States, Canada, and Japan.
Cohort B of the study, consisting of 112 intention-to-treat patients with a median age of 66.5 years, evaluated the specific triplet regimen of pomalidomide, dexamethasone, and daratumumab. All patients had received lenalidomide in the immediately prior line of therapy, and most patients (62.5%) had only one prior line of therapy. Overall, 28 patients (25%) had relapsed disease following lenalidomide, and 84 patients (75%) had disease that was refractory to lenalidomide.
Patients in cohort B received treatment in 28-day cycles until disease progression or unacceptable toxicity. Pomalidomide 4 mg/day was administered orally on days one to 21; dexamethasone 40 mg/day (20 mg/day in patients older than 75 years) was administered orally on days one, eight, 15, and 22; and daratumumab 16 mg/kg was administered intravenously on days one, eight, 15, and 22 of cycles one and two, days one and 15 of cycles three to six, and day one of cycle seven and beyond.
Trial Results
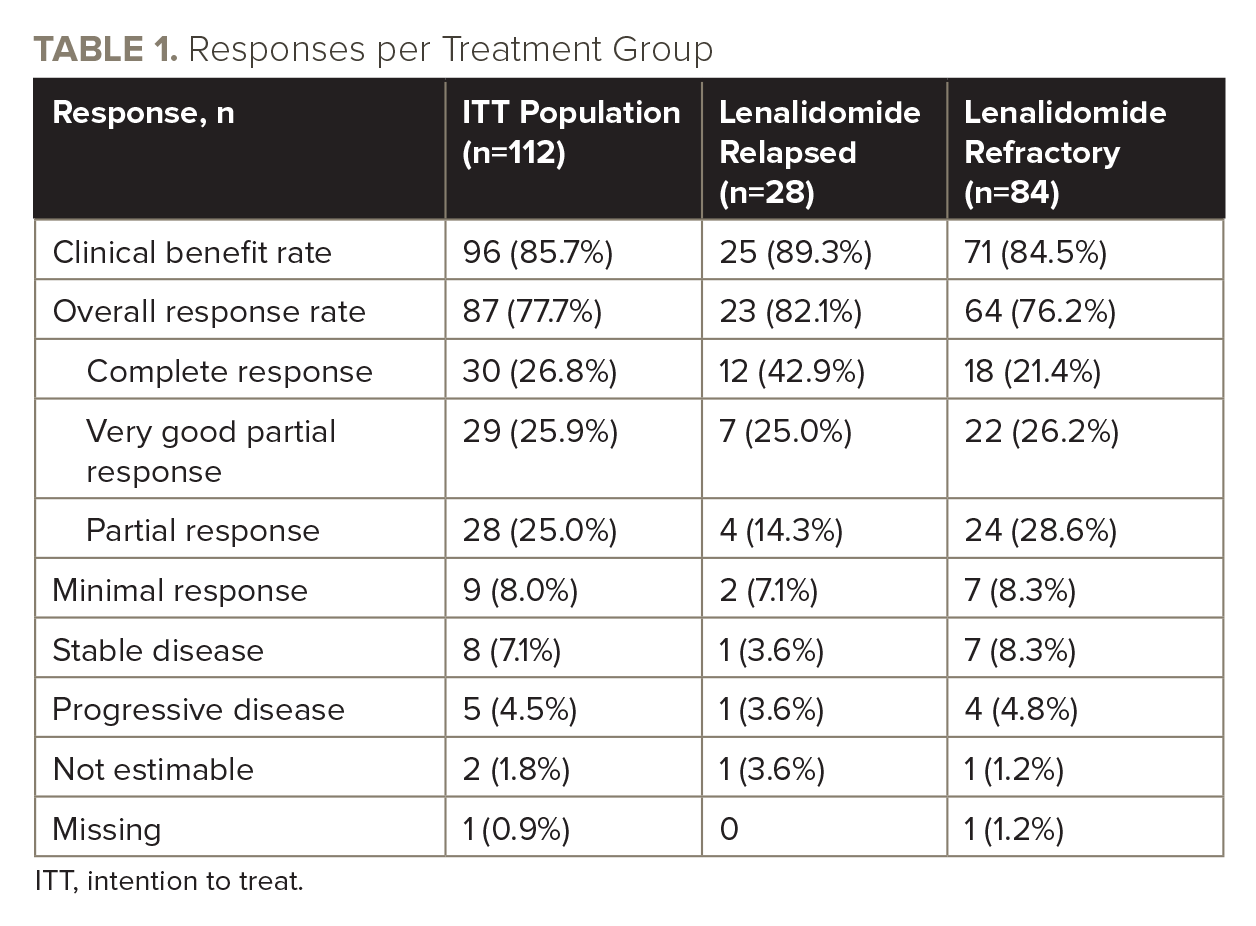
With a median follow-up of 28.4 months, the overall response rate in cohort B was 77.7% (see TABLE 1 for all responses), and median progression-free survival (PFS) was 30.8 months.
As of the data cutoff date, 31 patients (27.7%) remained on treatment. Among the 81 patients (72.3%) who had discontinued treatment, the most common reasons were progressive disease (n=46), withdrawal by patient (n=19), adverse events (n=7), transition to another commercially available treatment (n=2), and death (n=2). Treatment-emergent adverse events were experienced by 109 patients (97.3%), with the most common being infections (79.5%) and neutropenia (67.0%).
Immune-profiling analysis of cohort B during the first two cycles of treatment showed a marked increase in activated and proliferating natural killer and T cells and reduced naïve effector memory compartments, as well as depletion of CD38-expressing regulatory T cells with no increase in frequency of total regulatory T cells. These immune enhancements were also observed in patients with disease refractory to lenalidomide.
In this study, patients received pomalidomide and dexamethasone orally while daratumumab was administered intravenously. However, the authors noted that a subcutaneous formulation of daratumumab has recently been approved in the United States with indications for both newly diagnosed and relapsed/refractory MM, which is likely to offer quality-of-life benefits compared with intravenous infusions.
The study also observed either maintained or a trend toward improved health-related quality of life in patients receiving the triplet regimen. Using the EQ-5D assessment, mean changes from baseline through 12 cycles of treatment were stable, and at cycle 12, 26.1% of patients achieved minimum clinically important improvements in the EQ-5D index. Trends toward improvement were specifically seen in the areas of usual activities, pain/discomfort, and anxiety/depression.
“These updated findings in over 100 patients from cohort B of MM-014 reaffirm the benefit of combination pomalidomide, dexamethasone, and daratumumab in patients with early-line relapsed/refractory MM who have had prior lenalidomide, even in those with lenalidomide-refractory disease at first relapse,” the authors wrote. “The median PFS of 30.8 months observed in this study is the longest reported to date for a triplet regimen in a population of predominately lenalidomide-refractory patients.”
The study was sponsored by Celgene, a Bristol-Myers Squibb Company.
Reference
Bahlis NJ, Siegel DS, Schiller GJ, et al. Pomalidomide, dexamethasone, and daratumumab immediately after lenalidomide-based treatment in patients with multiple myeloma: updated efficacy, safety, and health-related quality of life results from the phase 2 MM-014 trial. Leuk Lymphoma. 2022;63(6):1407-1417.






 © 2025 Mashup Media, LLC, a Formedics Property. All Rights Reserved.
© 2025 Mashup Media, LLC, a Formedics Property. All Rights Reserved.