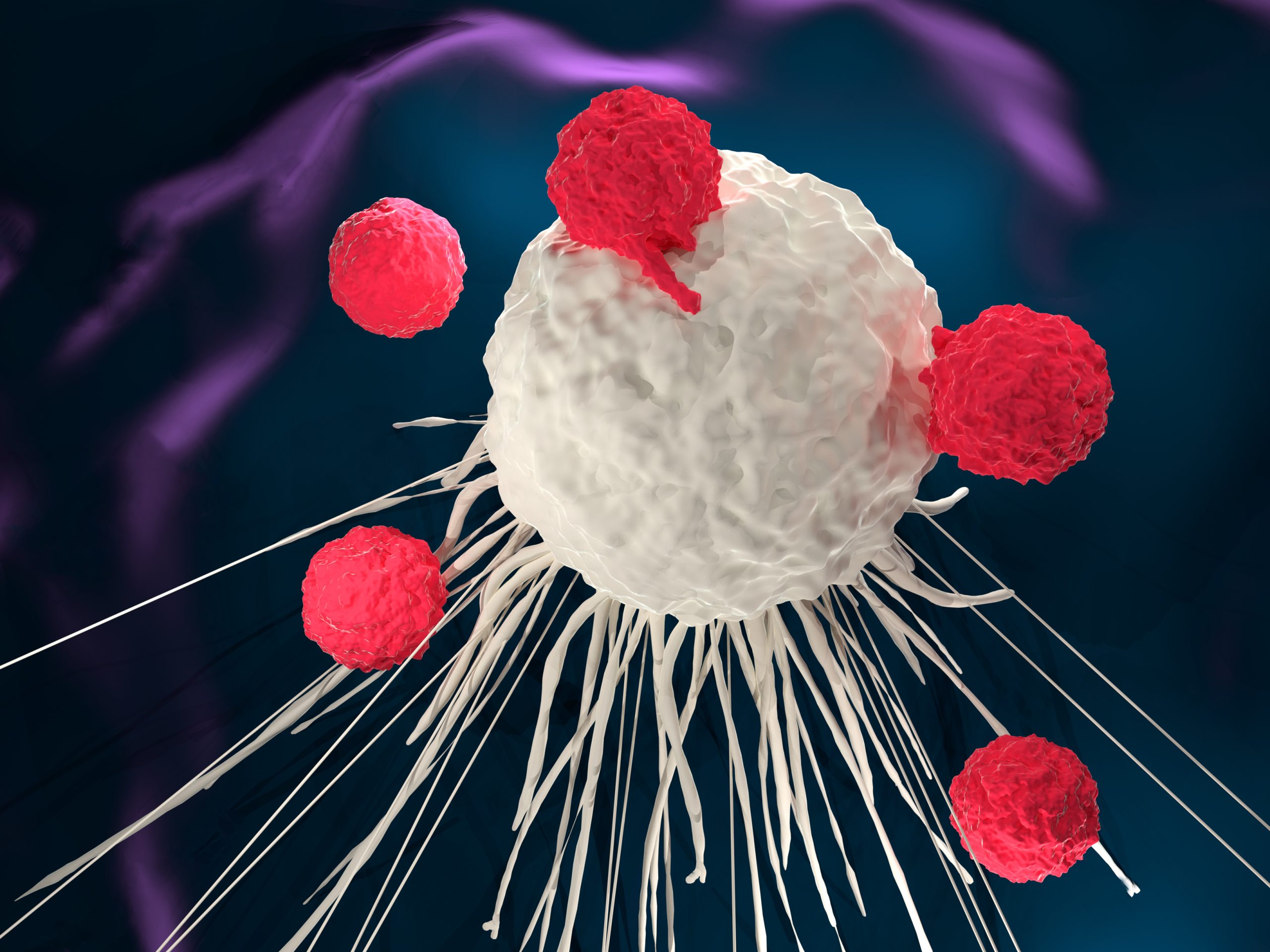
The European Commission has approved the chimeric antigen receptor (CAR) T-cell therapy tisagenlecleucel for the treatment of adult patients with relapsed or refractory follicular lymphoma who have received two or more lines of systemic therapy.
The approval was based on the results of the phase II ELARA trial that showed that tisagenlecleucel resulted in high response rates in these heavily pretreated patients. The overall response rate was 86%, and the complete response rate was 69%. Patients treated with tisagenlecleucel had prolonged durable response, with an estimated 87% of patients who achieved a complete response still in response at nine months or longer.
Half of patients treated with tisagenlecleucel experienced cytokine release syndrome after CAR T-cell infusion. No grade 3 or 4 events were reported. Neurological adverse events occurred in 9% of patients; 1% were grade 3 or 4. Severe infections occurred in 16% of patients.
The approval is applicable to all 27 European Union member states plus Iceland, Norway, and Liechtenstein.
This is the third approved indication for tisagenlecleucel in the European Union. It is also approved for pediatric and young adult patients with relapsed or refractory B-cell acute lymphoblastic leukemia and in adult patients with relapsed or refractory diffuse large B-cell lymphoma.
Source: Novartis Press Release, May 2022



 © 2025 Mashup Media, LLC, a Formedics Property. All Rights Reserved.
© 2025 Mashup Media, LLC, a Formedics Property. All Rights Reserved.